Conjunctival rhinosporidiosis in a temperate climate
Conjunctival rhinosporidiosis in a temperate climate
INITIAL PRESENTATION
Chief Complaint
Left medial canthal lesion for the past one month
History of Present Illness
A 27-year-old male patient was referred to the Oculoplastics clinic at the University of Iowa Hospitals and Clinics for evaluation of a left medial canthal lesion. The patient was unsure of the exact length of time the lesion had been present, but he first noticed it about one month prior to presentation. He reported that he had been unconcerned until a friend noted that it was getting bigger. The patient denied any blurriness or change in vision. He also denied pain, bleeding, drainage, or tearing. He presented with no other systemic symptoms, except for a recent upper respiratory infection. He denied any history of trauma, foreign travel, or close contact with animals. In retrospect, his history was notable only for swimming in stagnant water at a local rock quarry.
Past Ocular History: None
Past Medical History: None
Medications: None
Allergies: No known allergies
Family History: Non-contributory
Social History: Pertinent elements are detailed in the history of present illness.
Review of Systems: Negative except for what is detailed in the history of present illness.
OCULAR EXAMINATION
Visual Acuity with correction (Snellen)
- Right eye (OD): 20/15
- Left eye (OS): 20/15
Ocular Motility
Intraocular Pressure (IOP): (TonoPen)
Pupils
- OD: No relative afferent pupillary defect (RAPD)
- OS: No RAPD
External
- Right: Normal
- Left: Normal
Slit lamp exam
- Lids/lashes: Normal OU
- Conjunctiva/sclera
- Right: Clear and quiet
- Left: pedunculated vascular lesion arising from the superior nasal conjunctiva near the caruncle but not including it (Figure 1).
- Cornea: Clear OU
- Anterior chamber: Deep and quiet OU
- Iris: Normal architecture OU
- Lens: Clear OU
Differential Diagnosis
CLINICAL COURSE
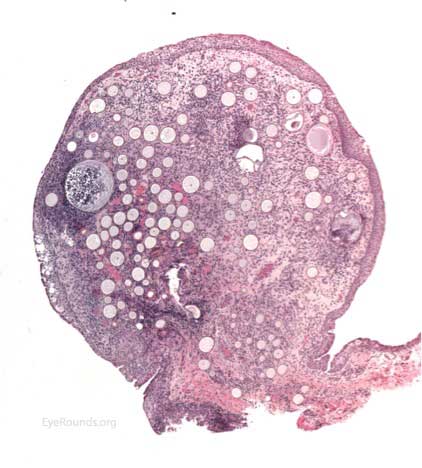
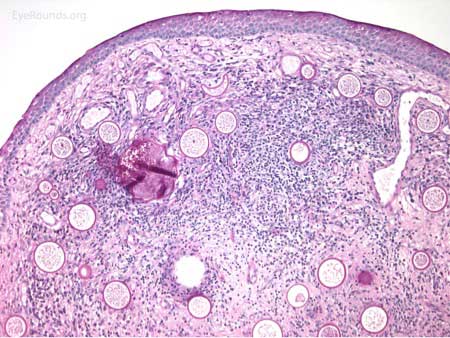
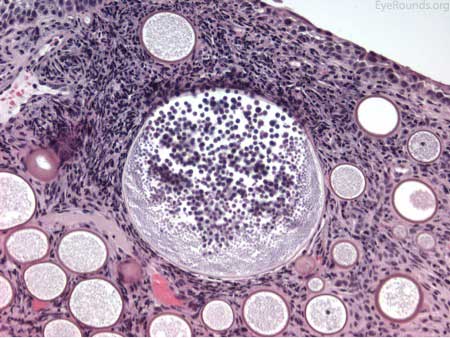
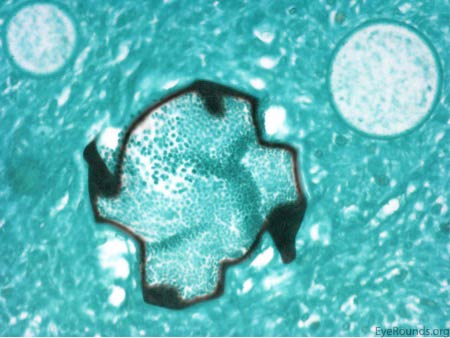
The patient underwent an excisional biopsy under local anesthesia. Microscopy revealed conjunctiva with mild epithelial hyperplasia and multiple sporangia in various stages of development and regeneration (Figure 2a). Inflammatory cells surrounded the sporangia, including neutrophils, lymphocytes, plasma cells, and multinucleated giant cells. Periodic acid-Schiff (PAS) stain highlighted the thick walls of the sporangia in various stages of the life cycle (Figure 2b). Mature sporangia revealed a surface pore with release of spores (Figure 2c). Gomori methenamine silver (GMS) stained the wall of the mature sporangium (Figure 2d). These findings were consisted with rhinosporidiosis.
The patient was seen in follow-up approximately 6 months later with recurrence of the lesion. At that time, repeat excision with cautery was performed. Consideration was given to dapsone treatment following the excision, but the patient declined. Follow-up 7 months later revealed no signs of recurrence.
DIAGNOSIS: Conjunctival Rhinosporidiosis
DISCUSSION
Etiology
- Rhinosporidiosis is an infection caused by Rhinosporidium seeberi. R. seeberi was thought to be a fungus for nearly 100 years but has more recently been classified as a Mesomycetozoea, an aquatic protozoan parasite (1,2). Rhinosporidiosis is endemic in parts of India and Sri Lanka, but quite sporadic in other parts of the world, including the United States (3,4). Additionally most of these cases have occurred in the warmer southern climates. Epidemiological evidence points to ground water as the source of R. seeberi, but culturing of R. seeberi in vitro from presumed contaminated sources has not yielded growth of the organism. Human-to-human transmission has not been documented, either, so it is not believed to contagious (2, 5).
Pathophysiology
- Local infection from exposure to the organism appears to be implicated in the pathogenesis of R. seeberi, but thus far, it has not been possible to intentionally infect an animal model (2). Infection usually causes a chronic granulomatous reaction resulting in polypoid growths within mucous membranes (5, 6). The nasal mucosa is most often affected, but 10-15% of cases are ocular. The most frequent ocular site of infection is the conjunctiva, followed by the lacrimal sac (2). There have been a few case reports of scleral melting and staphyloma formation secondary to R. seeberi infection (7).
Signs/Symptoms
- Chronic infection of mucous membranes by R. seeberi produces polypoid mass lesions commonly arising from the nasopharynx, oral cavity, or orbit/adnexa. The lesions are usually characterized by friability and abundant vascularity. Pinpoint white spots are also commonly seen on the surface which correspond with mature sub-epithelial sporangia (2).
Diagnosis
- Diagnosis relies entirely on histopathology which demonstrates trophocytes and sporangia in various stages of development and degeneration. Infiltration of acute and chronic inflammatory cells will also be present (5,6).
Treatment
- Rare cases of spontaneous regression have been recorded (2,3). However excision with local cautery is considered the most effective treatment (8). Cautery is used to reduce the risk of recurrence which is caused by endospores being released into nearby mucosa. Medical treatment with dapsone and amphotericin B remains controversial, as determining drug sensitivity has been impossible without the ability to grow R. seeberi in vitro. However, dapsone has had some success in treating rhinosporidioisis; it is believed to act by arresting sporangia maturation (9). Recurrence rates have been noted to vary by infection site with conjunctival and lacrimal sac recurrences being relatively low compared to nasopharyngeal recurrence rates (6).
Etiology
- Rhinosporidiosis is a chronic infection caused by Rhinosporidium seeberi, a Mesomycetozoea
- Endemic in parts of India and Sri Lanka, but there are only sporadic case reports in the United States
- Nasal mucosa is most commonly affected, but 10-15% of cases affect the conjunctiva and/or lacrimal sac
|
Diagnosis
- Diagnosis relies on histopathology
- Trophocytes and sporangia in various stages of development and degeneration will be seen
|
Signs
- Infection produces polypoid mass lesions, arising from the upper respiratory and/or orbital mucosa
- Lesions are characterized by friability, abundant vascularization, and pinpoint white spots on the surface
|
Treatment
- Excision with local cautery is considered the most effective treatment
- Systemic medication, especially dapsone, may be considered but efficacy is unclear
- Recurrence rates are relatively low in the conjunctiva and lacrimal sac
|
References
- Arseculeratne SN. Rhinosporidiosis: what is the cause? Curr Opin Infect Dis 2005;18(2):113-118.
- Arseculeratne SN. Recent advances in rhinosporidioisis and rhinosporidium seeberi. Ind J Med Microbiol 2002;20:119-131.
- Shrestha SP, Hennig A, Parija SC. Prevalence of rhinosporidiosis of the eye and its adnexa in Nepal. Am J Top Med Hyg 1998;59:2331-2334.
- Reidy JJ, Sedesh S, Klafter AB, Christopher O. Infection of the conjunctiva by Rhinosporidium seeberi. Surv Ophthalmol 1997;41(5):409-413.
- Harissi-Dagher M, Robillard N, Corriveau C, Mabon M, Allaire GS. Histopathologically confirmed ocular rhinosporidiosis in two Canadians. Can J Ophthalmol 2006;41:226-229.
- Rogers S, Waring D, Martin P. Recurrent lacrimal sac rhinosporidiosis involving the periocular subcutaneous tissues, nasolacrimal duct, and nasopharynx. Orbit 2012;31(5):358-360.
- Castelino AM, Rao SK, Biswas J, Gopal L, Madhavan HN, Kumar SK. Conjunctival rhinosporidiosis associated with scleral melting and staphyloma formation: diagnosis and management. Cornea 2000;19:30-33.
- Arora R, Ramachandran V, Raina U, Mehta DK. Oculosporidiosis in Northern India. Indian Pediatr 2001;38:540-543.
- Job A, Venkatesmaran S, Mathan M, Krishnaswami H, Raman R. Medical therapy of rhinosporidiosis with dapsone. J Layngol Otol 1993;107(9):809-812.
Suggested citation format:
Reddy AK, McAllister AR, Syed NA, Allen RC. Conjunctival rhinosporidiosis in a temperate climate. EyeRounds.org. posted November 3, 2015; available from https://eyerounds.org/cases/224-rhinosporidiosis.htm
last updated: 11/03/2015
Image Permissions:

Ophthalmic Atlas Images by EyeRounds.org, The University of Iowa are licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License.