
The University of Iowa
Department of Neurology and Department of Ophthalmology and Visual Sciences
Updated: November 18, 2016
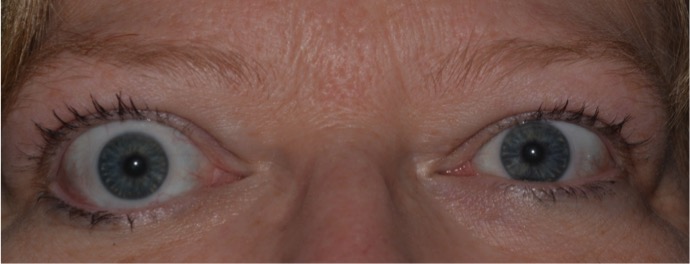
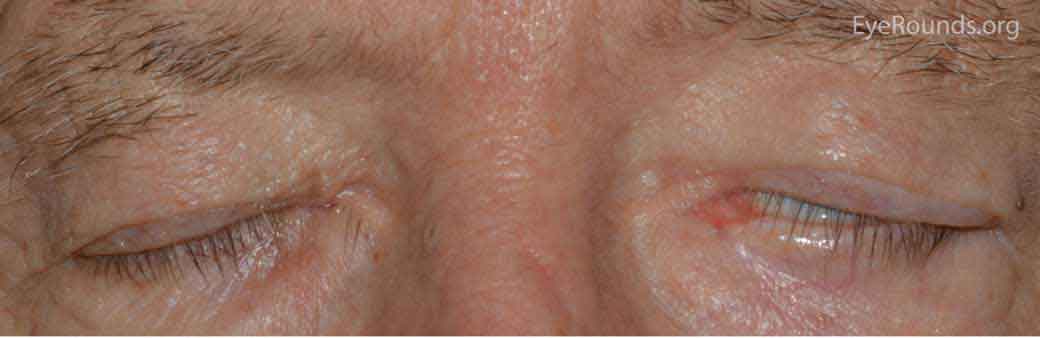
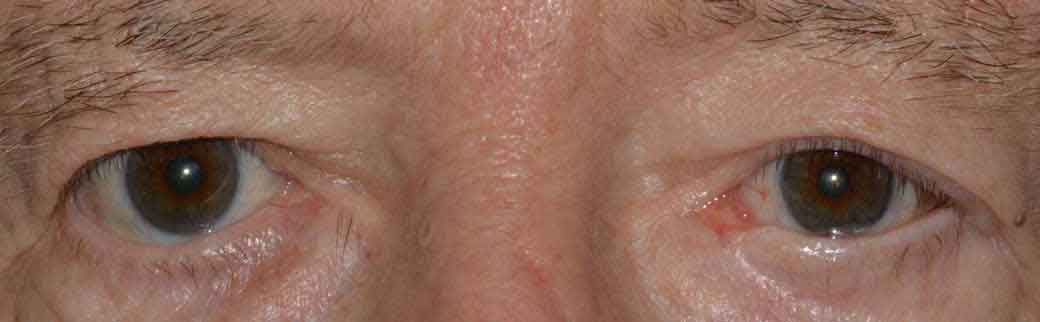
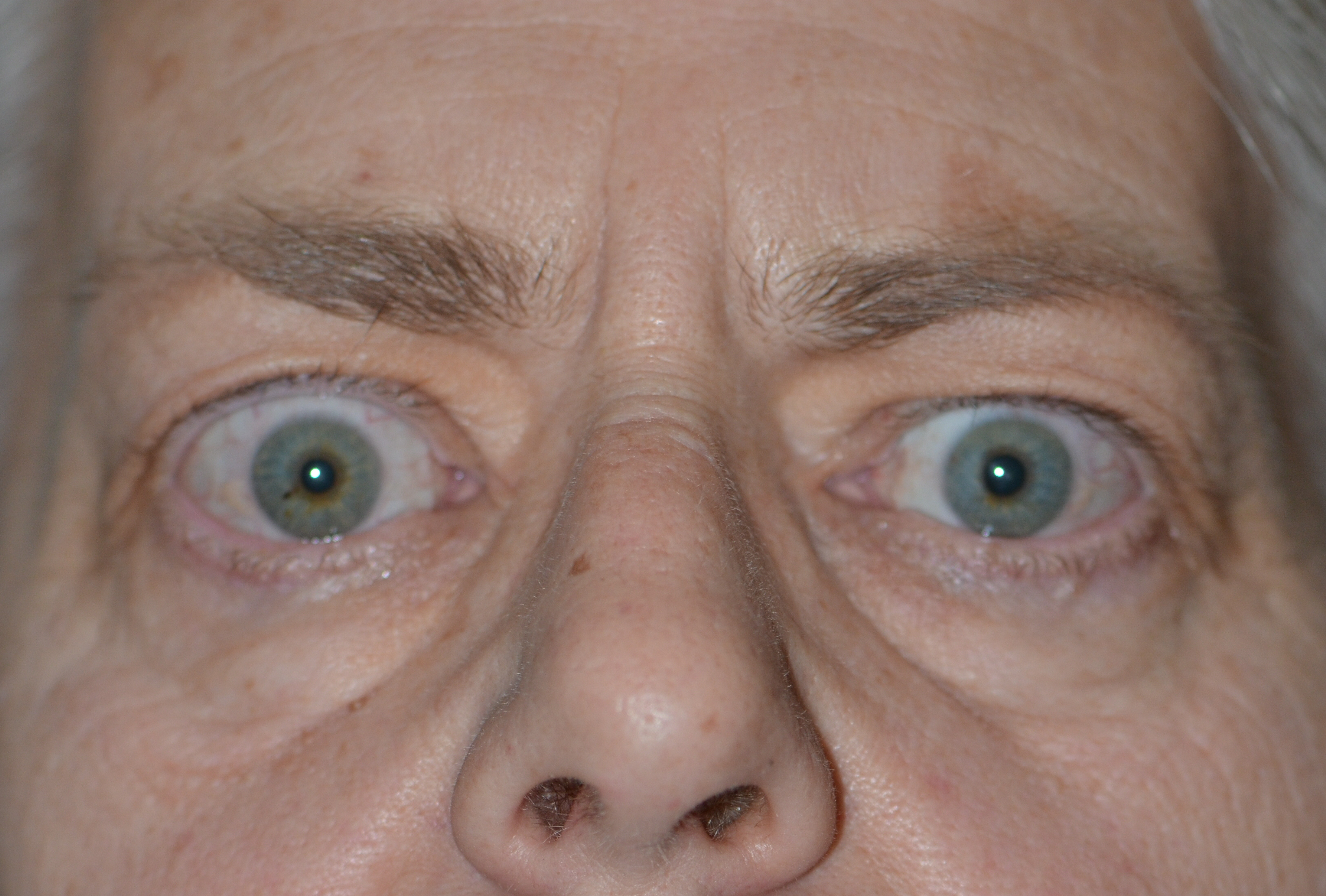
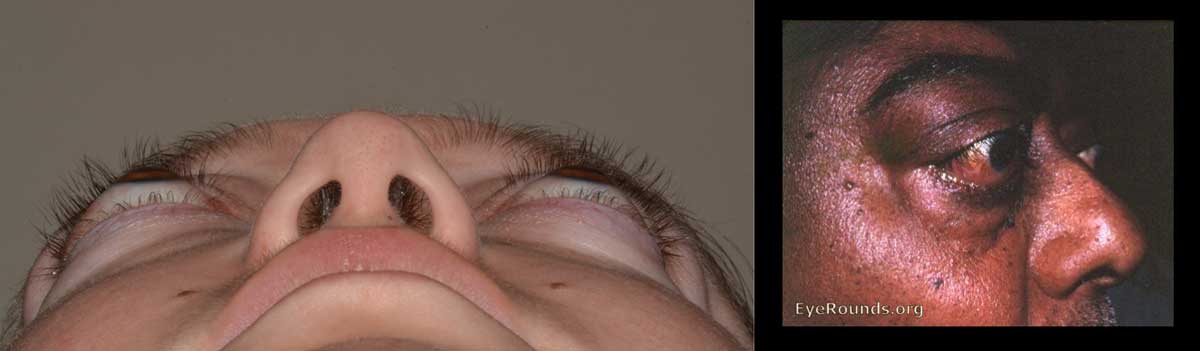
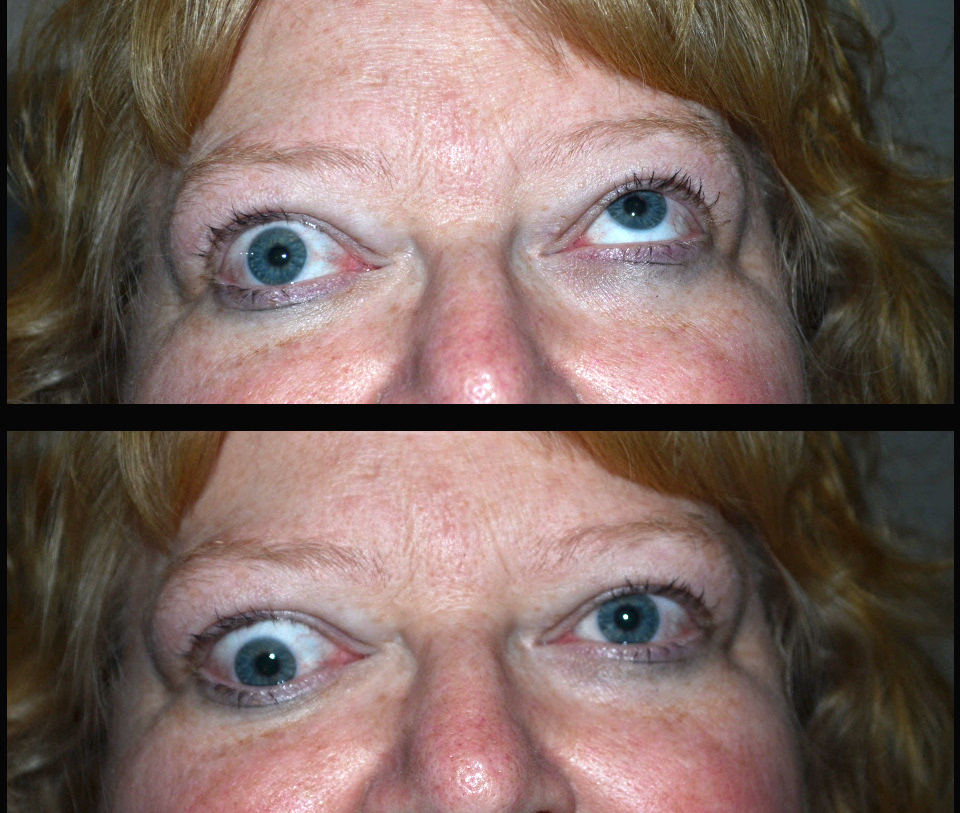
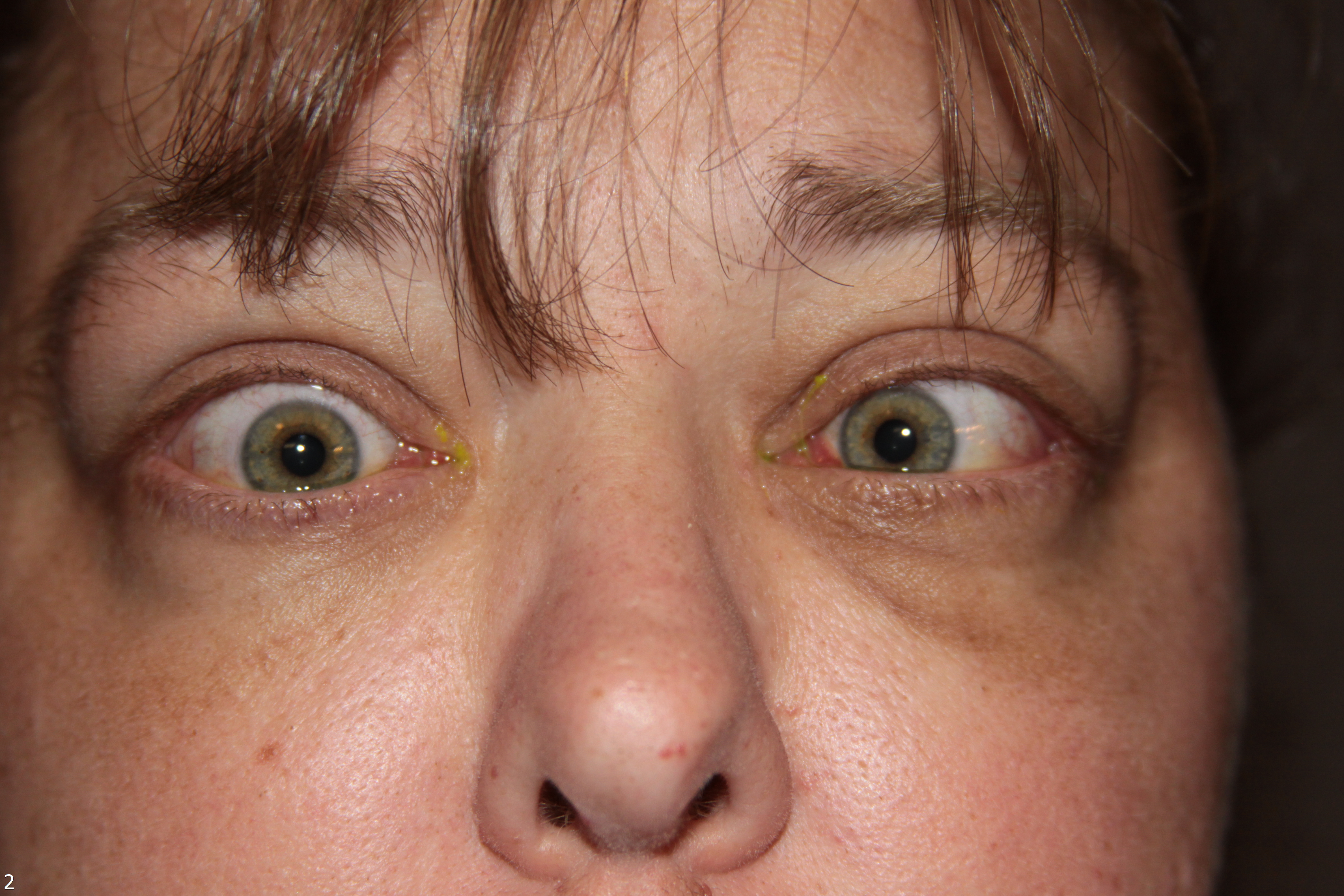
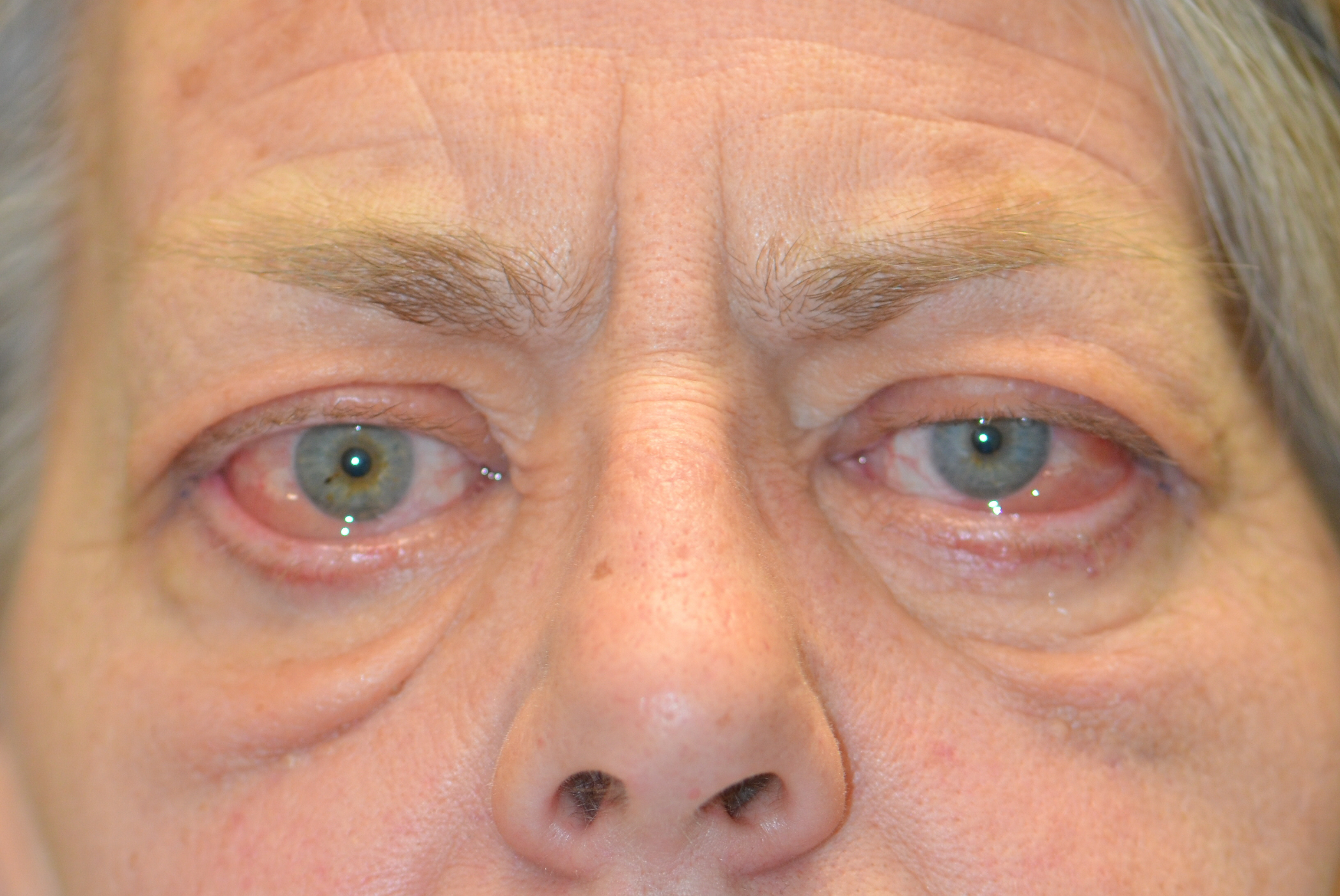
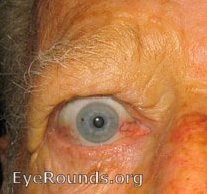
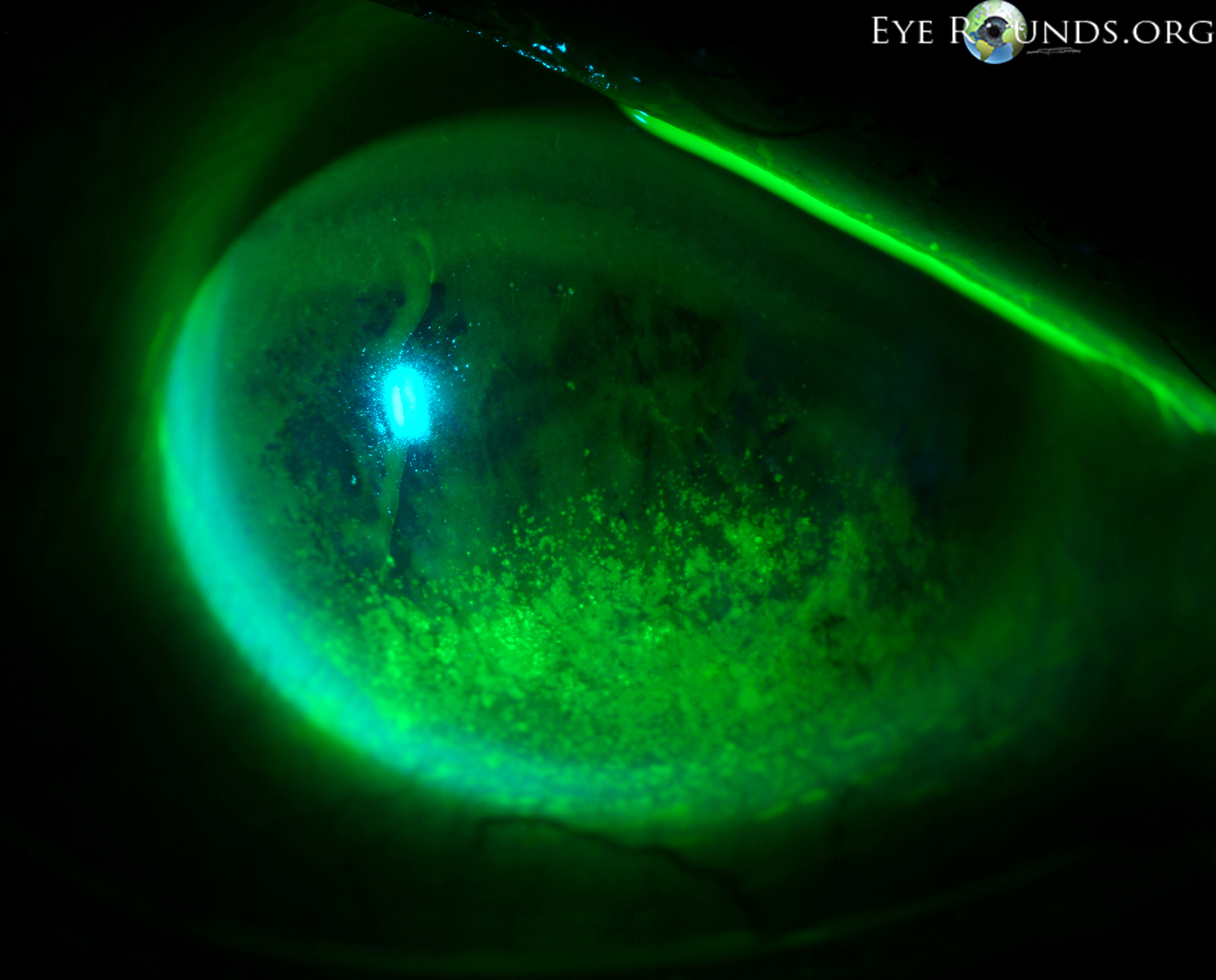
Thyroid eye disease (TED) is an autoimmune inflammatory disease of the eye and surrounding tissues. It is also recognized in the literature as Graves' ophthalmopathy, Graves' orbitopathy, thyroid-associated ophthalmopathy, and thyroid orbitopathy. TED was originally associated strictly with the Graves' triad of hyperthyroidism, pretibial myxedema, and eye disease. More recently, TED has also been noted in Hashimoto's thyroiditis as well as in the absence of a thyroid dysfunction. While symptoms are typically bilateral, they are often asymmetric. The most common presenting signs are orbital and periorbital edema, eyelid retraction, eyelid lag in downgaze, restrictive strabismus, compressive optic neuropathy, and exposure keratopathy with common symptoms of ocular irritation and dryness (Figures 1 and 2) [1]. The disease course of TED does not always coincide with thyroid activity or the treatment of underlying thyroid dysfunction.
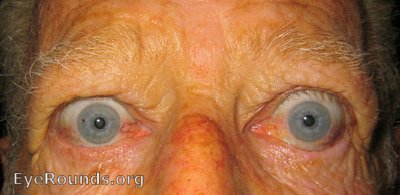
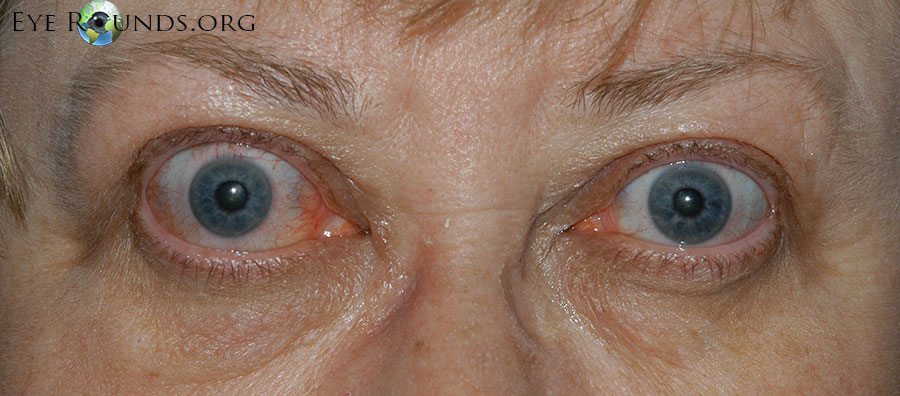
TED is the most common cause of orbital disease in North America and Europe and of both unilateral and bilateral exophthalmos. While TED is most commonly associated with Graves' disease, it can also occur in association with other thyroid states, pathologic or non-pathologic. TED has a higher prevalence in women than men (16 per 100,000 vs. 3 per 100,000, respectively). Both men and women demonstrate a bimodal pattern of age of diagnosis (40-44 and 60-64 years in women; 45-49 and 65-69 years in men). The median age of diagnosis is 43 years for all patients, with a range from 8-88 years. Patients diagnosed over the age of 50 years have a worse prognosis overall. Risk factors for TED include age, gender, ethnicity, and family history. A positive family history of TED is noted in 61% of TED patients [2].
TED exacerbation is thought to be associated with both genetic and environmental factors, such as cigarette smoking, low selenium levels, and stress [3]. Smoking has been shown to adversely affect the development, progression, and response to treatment of TED. Smokers are twice as likely to develop Graves' disease, and smokers who have Graves' disease are 7.7 times more likely to develop TED compared to nonsmokers with Graves' disease.
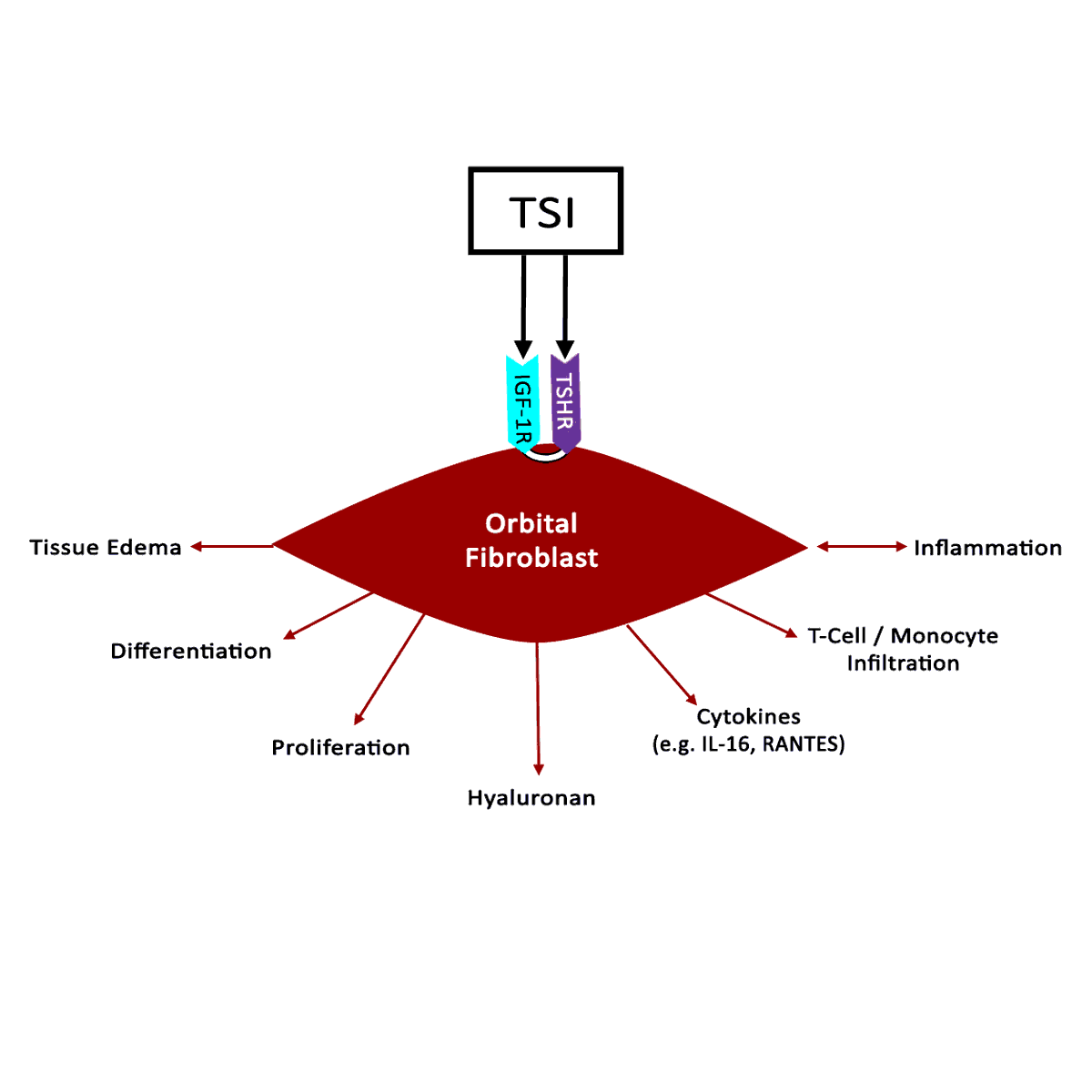
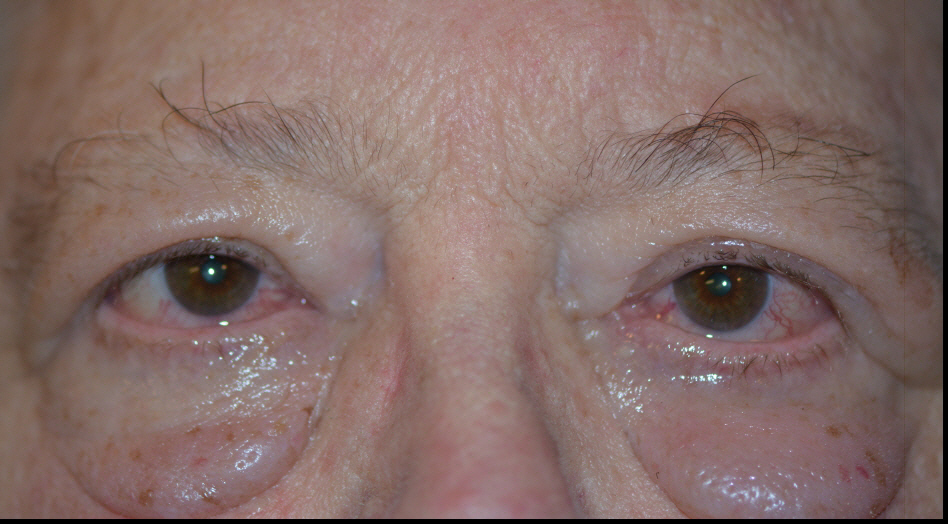
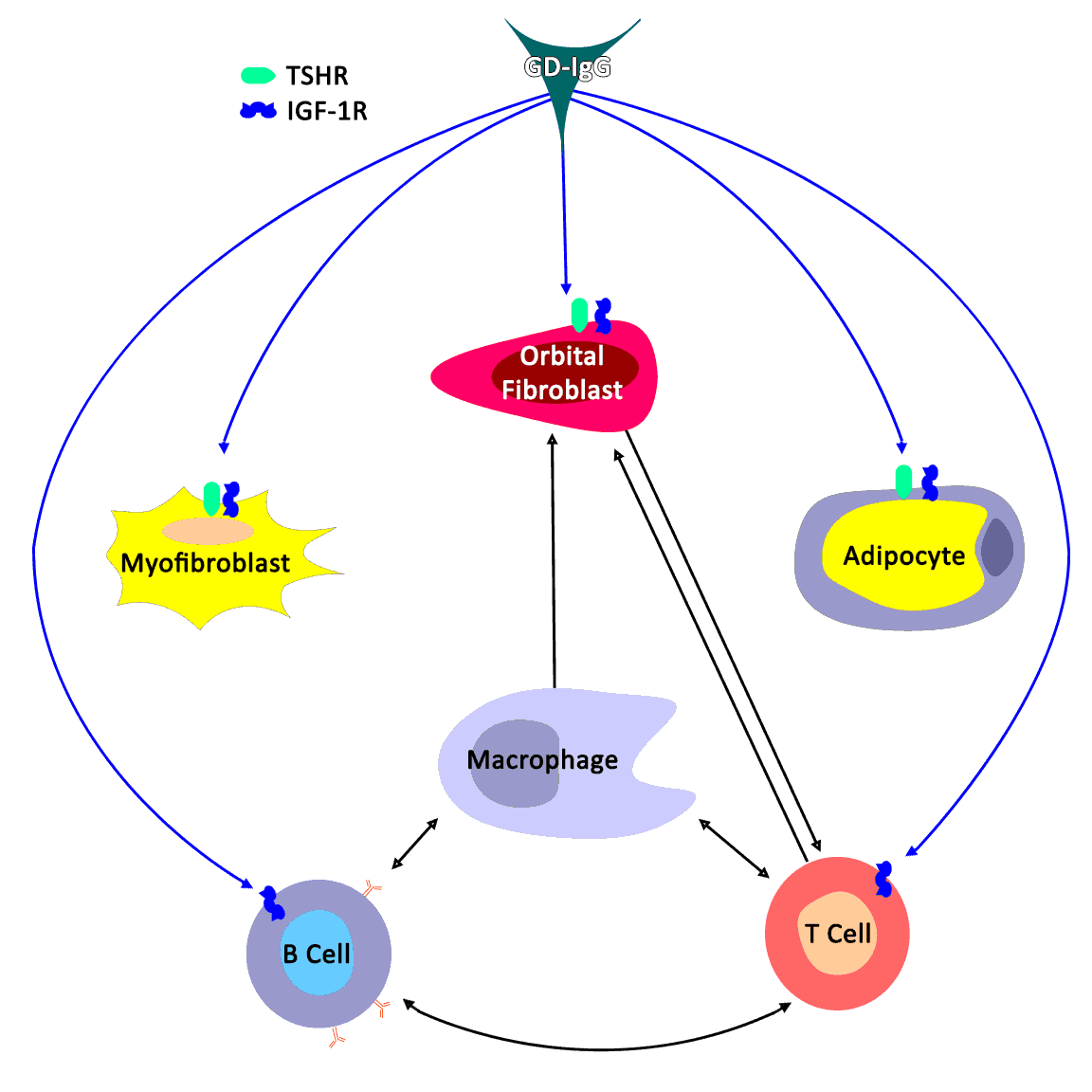
The thyroid state of a patient presenting with TED is quite variable: 90% hyperthyroid, 6% euthyroid, 3% with Hashimoto's thyroiditis, and 1% hypothyroid [2]. Patients are simultaneously diagnosed with TED and thyroid dysfunction 20% of the time, and 60% present within 1 year of onset of thyroid disease [9]. However, TED can present long before (up to 10 years) or long after (up to 20 years) the initial presentation of thyroid disease [2].
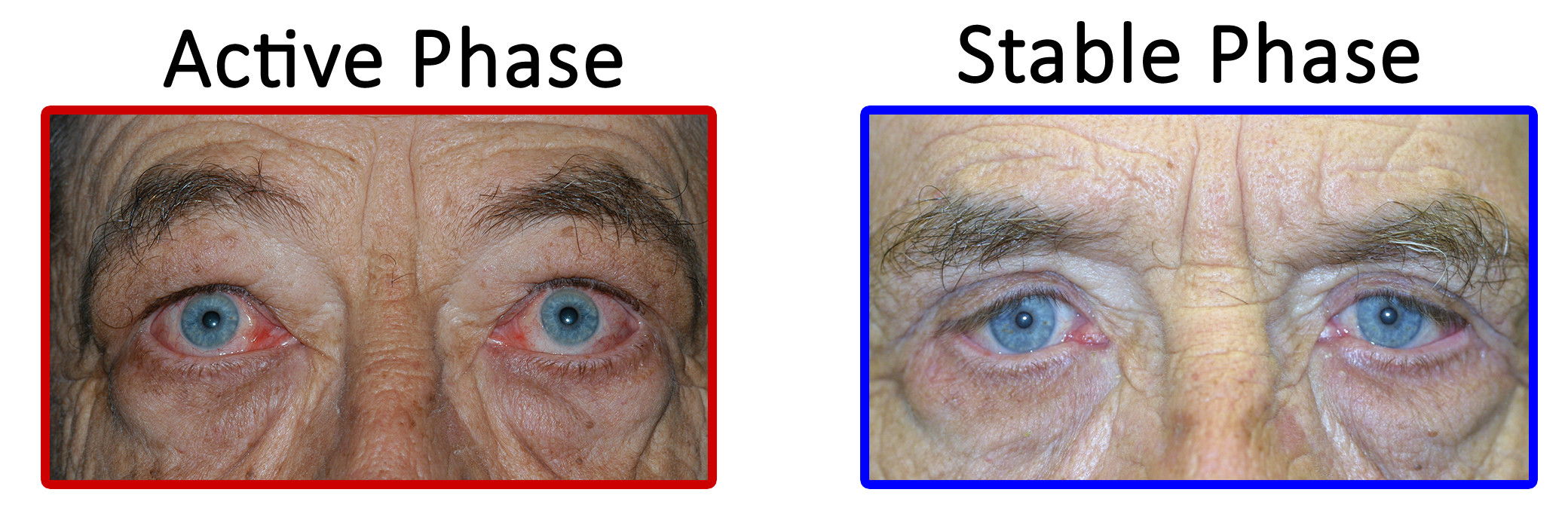
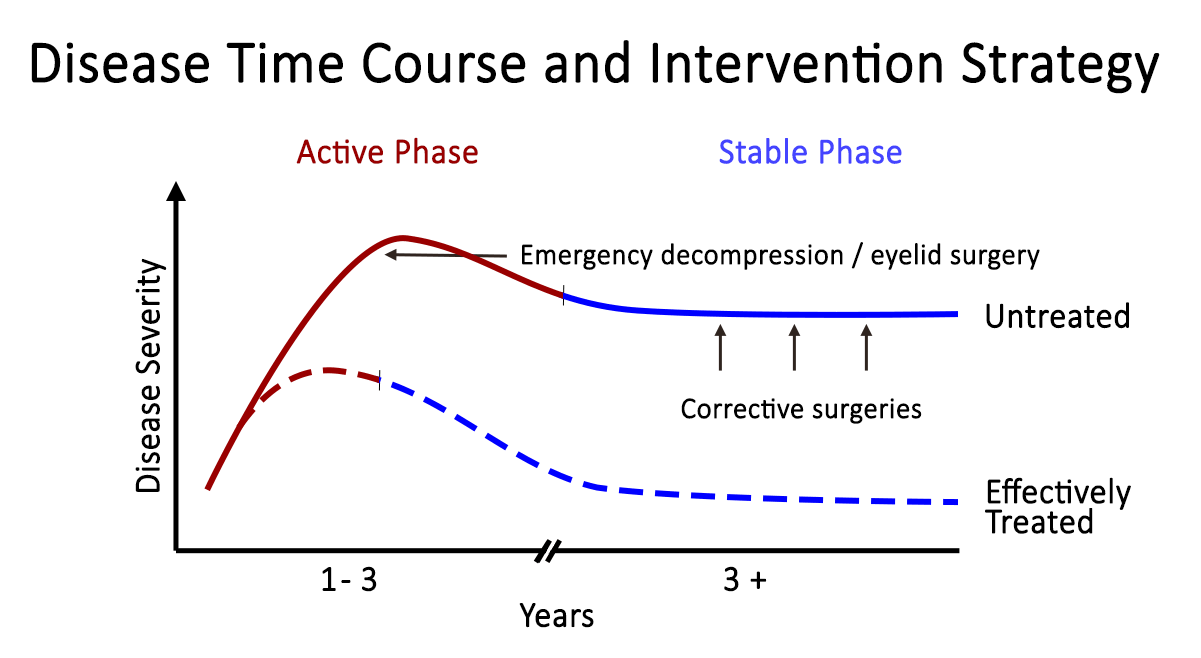
TED is a self-limiting disease and may present in one of two stages: active or quiescent (Figure 6). In the active stage, there is active inflammation, which can lead to orbital muscle enlargement, conjunctival injection and chemosis, ocular pain, and swelling of the periocular tissues and eyelids. This stage typically involves waxing and waning TED symptoms and can last months to years. On average, the active phase lasts for 1 year in non-smokers and 2-3 years in smokers. The quiescent phase follows spontaneous resolution of the active phase (Figure 7). Active TED has a recurrence rate of 5-10% but is less likely to recur after 18 months of quiescence [10].
When examining a patient with suspected TED, it is important to have a working differential. The following diagnoses share some similarities to the clinical presentation of TED
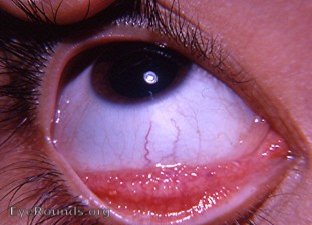
Allergic conjunctivitis– While both can cause excess tearing and conjunctivitis, allergic conjunctivitis tends to be acute in onset from a new exposure, causes itching, can have papillary conjunctival reaction, and is not associated with eyelid retraction or exophthalmos (Figure 18).
Myasthenia gravis (MG) – Like TED, MG patients can present with diplopia. However, MG tends to worsen throughout the day and improves after rest while diplopia in TED is not typically variable. Also, MG patients may present with ptosis, which is not associated with TED. Diplopia associated with TED is restrictive in nature, which can be determined by forced duction testing.
Orbital myositis (OM)OM causes enlargement and inflammation of the muscle body and tendon insertion, rather than just the muscle body, as is the case in TED patients. Orbital myositis is not generally associated with eyelid retraction. OM is usually unilateral. A bilateral presentation would be unusual for OM, whereas TED can present either way.
Orbital tumors – Orbital tumors are typically unilateral in presentation and can cause proptosis and a wide variety of motility disturbances depending on location. Orbital tumors are unlikely to cause eyelid retraction or lid lag. (see Eye Rounds case on Cavernous Hemangioma)
Carotid-cavernous fistula (CCF)– Patients may hear pulse-synchronous tinnitus. Presentation may include proptosis, pulsatile exophthalmos, dilated conjunctival and episcleral vessels, elevated intraocular pressure, or enlarged EOM depending on the amount of flow through the fistula and the degree of congestion. A CCF would not cause eyelid retraction or temporal flare. (see Eye Rounds case on CCF)
Chronic progressive external ophthalmoplegia (CPEO)– CPEO slowly progresses over 5-15 years with most patients presenting with ptosis. All cardinal directions of gaze are affected, with downgaze most likely spared. TED, conversely, typically affects downward and nasal gaze.
Inflammatory orbitopathy, such as granulomatosis with polyangitis (GPA, formerly known as Wegener's granulomatosis) – GPA typically presents with a mix of upper airway, lower airway, and renal pathologies. Patients may have conjunctivitis, episcleritis, scleritis, and/or uveitis. Other than conjunctivitis, these findings are uncommon in TED patients.
IgG4 disease – Tumefactive lesions and fibrosis affecting one or more organs characterize this fibro-inflammatory disorder. It is most commonly present in the biliary tree, retroperitoneum, salivary glands, orbit, and lymph nodes. It is thought to involve both humoral and cell-mediated immunity. Orbital IgG4 disease often involves painless swelling of the extraocular muscles, lacrimal glands, and infraorbital nerves in combination with paranasal sinus disease. IgG4 disease can also present as an inflammatory orbital mass lesion.
In diagnosing TED, two of the following three clinical requirements must be met [13 ,14]
If TED is suspected, one must determine disease activity and severity in order to assess the urgency of treatment.
In assessing the activity level of TED in a patient, the clinical activity score (CAS) can be used [15].
Figure 19. Clinical Activity Score
Initial Visit( 1 point each)
CAS ≥ 3 → "Active"
Follow-up Visit (1 point each)
- Criteria 1-7
CAS ≥ 4 → "Active"
In classifying the severity of TED, 3 indices are typically used
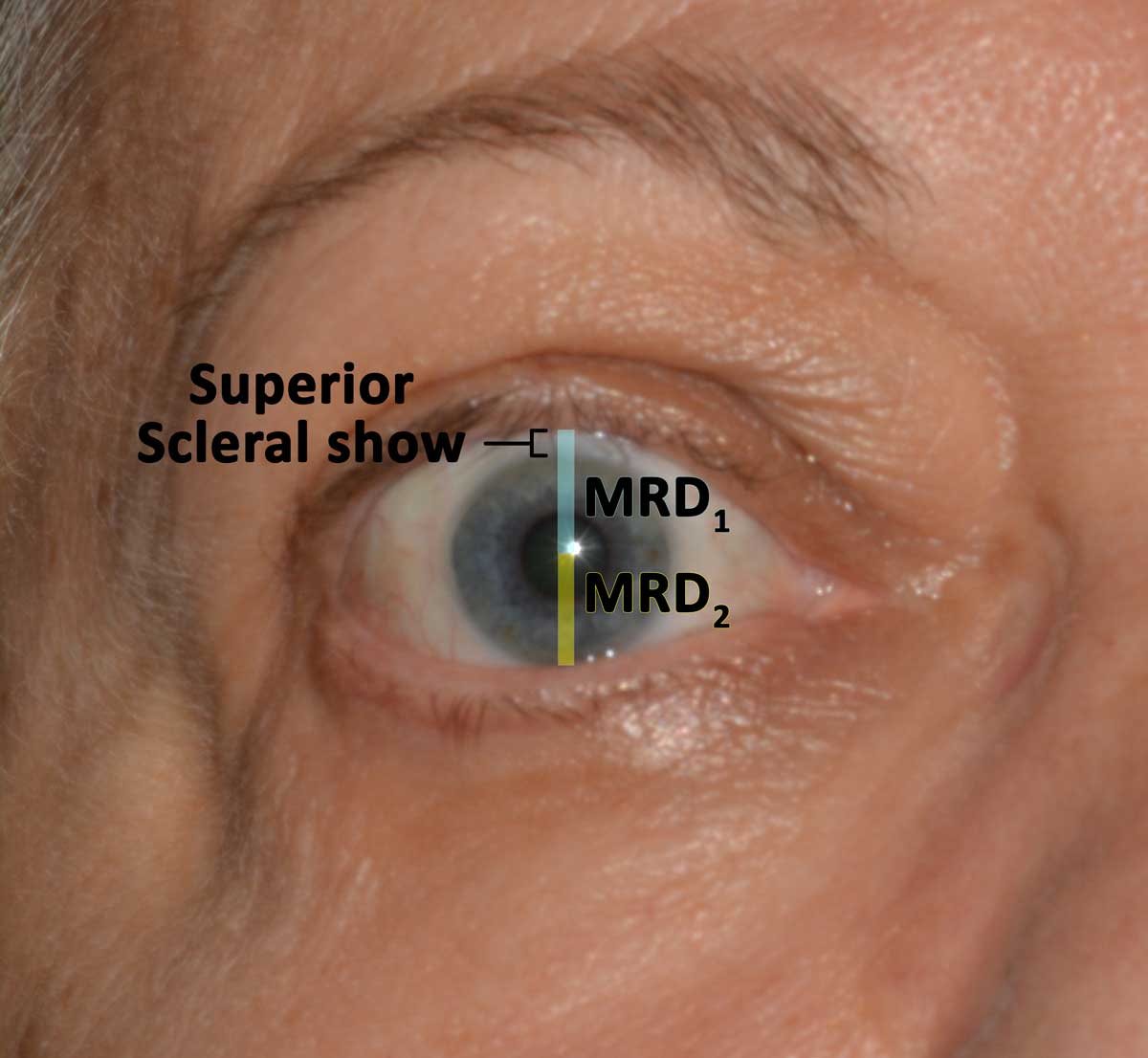
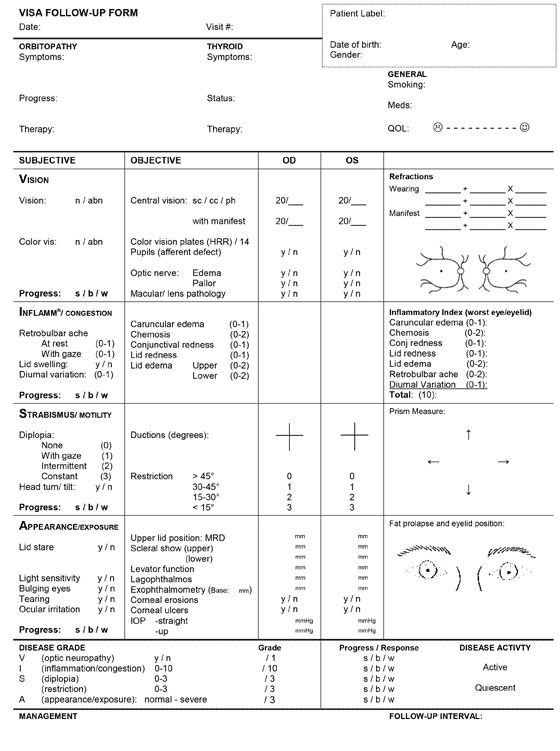
Management goals include maintenance of general health and well-being, achieving a euthyroid state (without post-treatment hypothyroidism), and promotion of smoking cessation. Both smoking cessation and euthyroidism help prevent further exacerbation and decrease the duration of active disease. From an ophthalmologist's perspective, the primary goal is to preserve visual function, while also preventing exposure keratopathy, correcting diplopia, and improving blink dynamics and cosmesis.
TED is a self-limiting disease, with patients moving from the active to quiescent phase within 1-3 years with a 5-10% risk of recurrence [10] Treatment for TED should start at the time of the diagnosis, as treatment becomes less effective as the disease progresses from the early, acute, active phase to the chronic quiescent phase.

Aside from threatening vision and causing ocular and orbital pain, TED can be disfiguring and emotionally and psychologically taxing for many patients. Waxing and waning symptoms can be frustrating for both patient and provider. Education and reassurance are integral components of patient care. Peer support groups are invaluable for many patients.
The following section starts with an overview of managing hyperthyroidism, followed by the different treatment options used in TED. It concludes with a discussion about therapeutic modalities specific to each sign or symptom associated with TED.
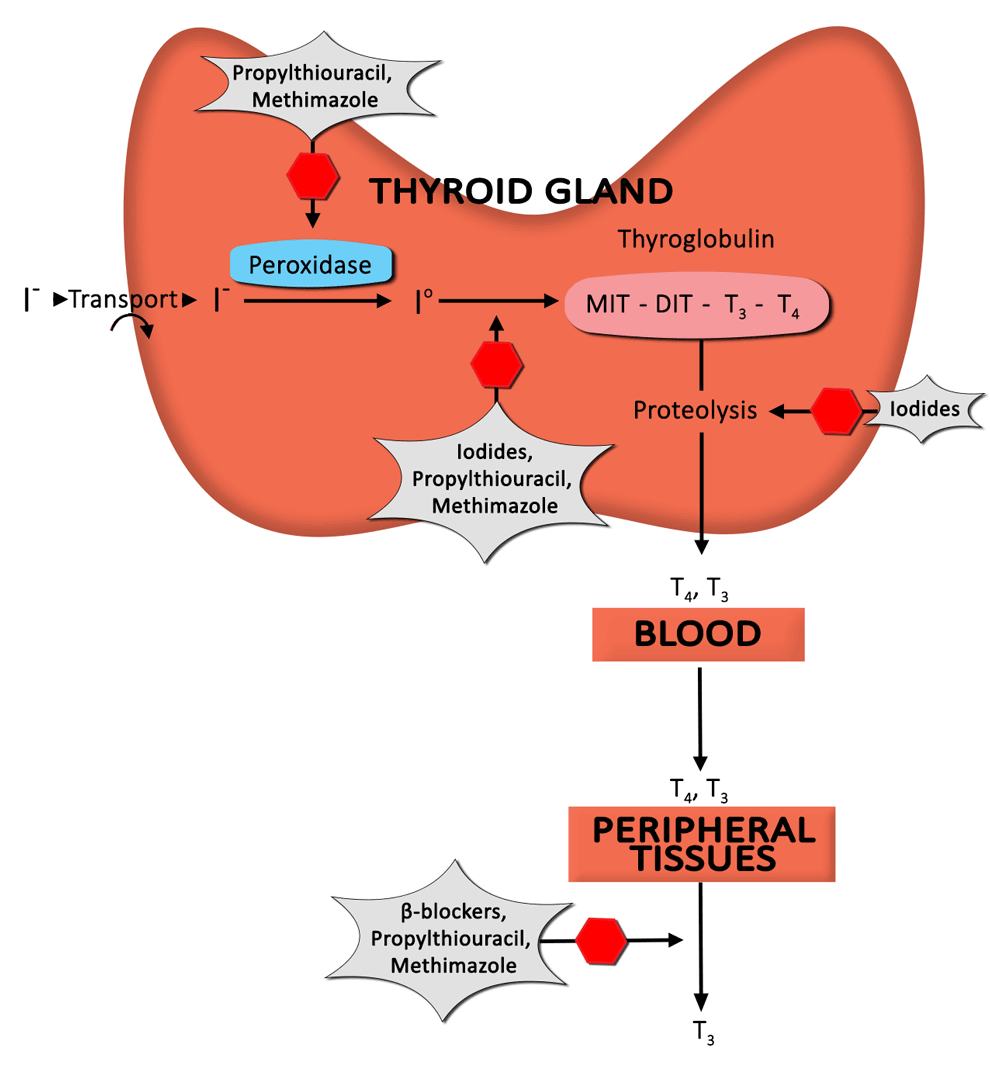
Corticosteroids are a mainstay of treatment in TED. The benefit derived from corticosteroid administration is due to anti-inflammatory and immunosuppressive effects. Unfortunately, a significant percentage of patients respond only partially (or not at all), and recurrences upon dose reduction or cessation are not infrequent [19].
Orbital radiotherapy (ORT) has been used in the management of TED for nearly a century and can be used alone or in conjunction with corticosteroids [26 ,27].
Overview (Figure 24)


Treatment Modalities


Suggested citation format
Liaboe, CA, Clark TJ, Carter KD, Shriver EM. Thyroid Eye Disease (TED): An Introductory Tutorial and Overview of Disease. EyeRounds.org. November 18, 2016; available from https://eyerounds.org/tutorials/thyroid-eye-disease/index.htm