Chief Complaint: Blurred "fuzzy" vision in the left eye (OS), gradually worsening over the past two weeks.
History of Present Illness:The patient is a systemically healthy 42-year-old Caucasian female prison inmate. She began to notice that her vision was fuzzy and dark over the past two weeks. The changes have been painless, and the eye has not become red or irritated.
Past Ocular History: Unremarkable for any past ocular surgery or trauma. No contact lens use. History of "uveitis", OS, in 1987. On further questioning, her prior episode of ocular irritation was preceded by mild flu-like symptoms.
Medical History: No other health issues. Recent PPD and HIV testing was negative.
Medications: None
Family History: Non-contributory
Social History: Prison inmate.
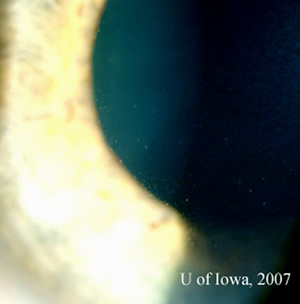
| 1A: Slit lamp view, OS, focused on the cornea demonstrates fine keratic precipitates. | 1B: Vitreous inflammation obscures the details of the posterior pole. |
 |
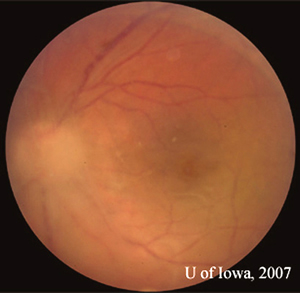 |
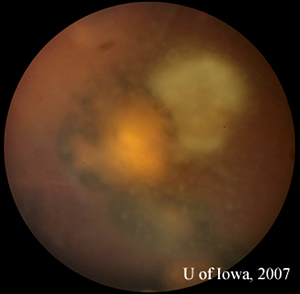
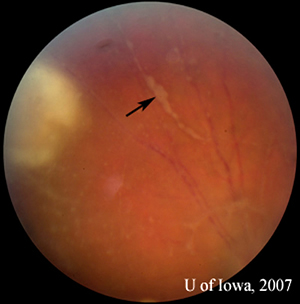
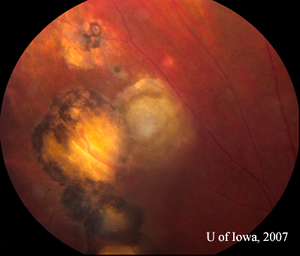
| 2A: Close view of lesion, OS. A unifocal area of inflammation is seen adjacent to a chorioretinal scar. | 2B: Thick peri-arteriolar Kyrieleis plaques are evident (arrow) on vessels near the active Toxoplasmosis lesion. |
 |
 |
Course: This patient presents with symptoms and a clinical picture consistent with toxoplasmosis retinitis. Treatment with 800/160 Trimethoprim/Sulfamethoxazole (Bactrim DS) was initiated promptly, as well as topical prednisolone and cycloplegic drops. Meanwhile, additional laboratory studies were performed to confirm the suspicion of toxoplasmosis and address less likely alternative causes, including syphilis, sarcoidosis, and leukemia or lymphoma. Tests for syphilis (RPR/FTA) were non-reactive. A complete blood count (CBC) and differential were normal, chest X-rays were clear, and the serum angiotensin converting enzyme (ACE) level was within normal limits. As noted by history, a recent tuberculosis skin test (PPD) and human immunodeficiency virus (HIV) serology were negative. Elevated toxoplasmosis IgG levels were confirmed, though the IgM antibody was not detected.
The patient returned six days after initiating antibiotic treatment for further evaluation. Her visual acuity had improved slightly to 20/160 in the affected eye and both the anterior and posterior segment inflammation had begun to subside. Oral prednisone was initiated at 40mg daily. The oral Bactrim DS was continued, as was topical prednisolone and cycloplegic drops. Slow visual improvement continued and the whiteness and elevation of the retinal lesion began to subside into a pigmented chorioretinal scar resembling the older, adjacent ones (see Figures 4A and 4B). At follow-up in July of 2007, vision had improved to 20/125 and inflammation was significantly reduced (see Figure 3). A careful taper of oral and topical steroid was initiated. Plans were made to continue Bactrim DS until the steroid taper was completed.
| Five weeks after initiating therapy, the right eye remained quiet. Inflammation in the left eye had subsided substantially (compare to inflammation seen in Figure 1B). |
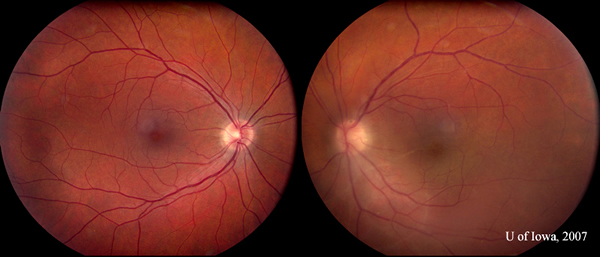 |
| 4A: July 2007 montage, OS, reveals reduced inflammation. A string of adjacent scars is evident, suggestive of multiple previous recurrences. | 4B: Closer view of the toxoplasmic lesion, OS, after six weeks of therapy. The previously active lesion appears to be involuting into a pigmented scar. Perivascular infiltrates have resolved (compare Figure 2B). |
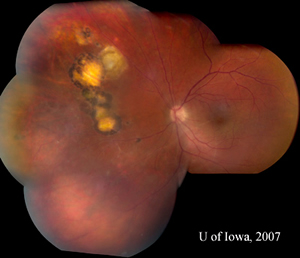 |
 |
Toxoplasma gondii is a parasitic protozoa that is probably the most common cause of posterior uveitis. This intracellular protozoa is ubiquitous. In the United States, it is frequently associated with a feline carrier. Toxoplasma oocysts are shed in large numbers in the feces of infected domestic cats and in some cases, in wild felines, such as bobcats and leopards (O'Connor 1974). The shed oocyte may retain its infectious capacity for up to a year. An active oocyte (filled with sporozoites) thus contracted from outdoor sand or soil (or from changing a litter box) may invade the tissues of an intermediate host, such as the human. Alternatively, the cyst form of the organism (filled with bradyzoites) may enter the human who consumes cyst-infected undercooked meat of another infected, intermediate host (especially lamb or pork and, less commonly, beef). If favorable reproductive conditions are present, the ingested cyst or oocyte may release thousands of bradyzoites or sporozoites, respectively, which transform into actively dividing tachyzoites. This active form may proliferate within any nucleated cell of the infected human host. Thus protected from host (serum) antibody, the tachyzoite can proliferate within the host cell until the swollen cell ruptures, exposing the organism temporarily to humoral immunity. It has been hypothesized that this lifecycle may explain why the disease course activates or progresses despite high serum antibody levels against the organism (O'Connor, 1970). As the body mounts a successful campaign against the active tachyzoites, tissue cysts containing bradyzoites may begin to appear, especially within the retina and other central nervous system tissues. These organisms may lay dormant for years, before some undefined stressor or condition prompts recurrent activity of disease adjacent to prior scars.
Presentation: In the human eye, toxoplasma infection creates a necrotizing chorioretinitis with varying degrees of posterior and anterior uveitis. Active chorioretinal lesions have a "fluffy" yellow-white appearance with indistinct margins and represent areas of focal, necrotizing disease. There is often a focal vitreous infiltrate overlying and surrounding the retinal lesion. At other times, the vitritis may be impressive, creating the "headlight in the fog" described in posterior uveitis. The presence of an active, unifocal area of acute chorioretinal inflammation adjacent to an old chorioretinal scar is highly suggestive of toxoplasmic chorioretinitis. Old lesions are often pigmented and clustered or linearly arranged across the fundus, suggestive of episodic new eruptions of acute inflammation arising from adjacent scarring (see Figure 4A). Perivasculitis may be present in vessels near the active lesion. In some cases, the periarteriolar collections of cells may be seen as distinctive, lobular Kyrieleis plaques (see Figure 2B). Anterior iridocyclitis sometimes accompanies the chorioretinitis. Usually this is moderate, but it can at times be severe and granulomatous in nature. Inflammation may last weeks to months and will usually resolve spontaneously in the immunocompetent host.
Treatment: Non-sight-threatening lesions are frequently managed with observation. This is especially true in the pregnant female, for example, to avoid the potential risk of systemic multi-agent antibiotic therapy. However, in the non-gravid patient where the optic nerve or macula are threatened or the vision is significantly restricted (i.e. below 20/70), therapy should be instituted. Treatment includes an intravitreal dose of clindamycin (1 mg in 0.1 ml) followed by prompt initiation of oral antibiotics (see below) as well as topical anti-inflammatory and cycloplegic agents. This is followed, at least 24-48 hours later, by the initiation of oral prednisone at high doses that are tapered slowly. Intravitreal triamcinolone and dexamethasone are contraindicated, as intraocular steroids typically cause widespread toxoplasmic retinitis.
The "classic" antibiotic "triple therapy" is 5-6 weeks of oral pyrimethamine, sulfadiazine, and folinic acid (Giles 1964, Engstrom, 1991). This regimen is often given as two 50 mg loading doses of pyrimethamine, 12 hours apart, followed by 50 mg daily (divided into twice daily dosing). Sulfadiazine may be given as a 2 g loading dose, then 1 g orally four times a day. Because these antibiotics interfere with folic acid metabolism, patients should take 3-5 mg of oral folinic acid supplementation twice a week while on the antibiotics in order to reduce the risk of thrombocytopenia and leucopenia (Giles, 1964).
Combination therapy, as listed above, may be hard to obtain and other antibiotics have been used with success in ocular toxoplasmosis. These include clindamycin (i.e. 300 mg four times daily) used alone (Lakhanpal, 1983) or in combination with triple therapy (Tabbara 1980). Another medication that has been used is the combination of trimethoprime-sulfamethoxazole in double-strength dosing (also known as Bactrim DS, 800-160 mg) with or without clindamycin (UpToDate, 2005 and Bonfioli, 2005). At the University of Iowa, we have found these regimens to be available, very effective, and well-tolerated. Sulfadiazine and trimethoprime-sulfamethoxazole should not be used in a patient with an allergy to sulfa drugs. A review of a variety of alternative antibiotic treatments utilized and the associated doses is available in the literature but is beyond the scope of this article (Bonfioli and Orefice, 2005). For ocular toxoplasmosis, there has not been a randomized, clinically-controlled study to demonstrate the superiority of one antibiotic regimen over another. This was pointed out in a Cochrane Review of current research on the topic (Gilbert RE, et al, 2002).
Most clinical sources agree that antibiotics should be initiated concurrent or before (and continued throughout) the administration of oral steroid therapy. Though some have reported the use of oral corticosteroids alone, we are concerned by reports of severe fulminant disease that has developed on oral steroid therapy alone (Nicholson, 1976 and Sabates, 1981) which would suggest that this is not recommended.
Surprisingly, ELISA testing for anti-Toxoplasma antibodies in the U.S. has revealed seropositivity in 20-70% of the population. It is estimated that acute infection goes unrecognized by 80-90% of individuals. In others, a mild to moderate flu-like or mononucleosis-like prodrome may accompany the initial infection.
In the immunocompromised host (i.e. HIV-positive), the necrotizing chorioretinitis may be so severe as to resemple acute retinal necrosis (ARN, or necrotizing herpetic retinitis). Neuroimaging is indicated in patients with AIDS because CNS toxoplasmosis lesions have been reported in up to 29% of AIDS patients with retinal lesions. In these patients, treatment must be aggressive and include life-long suppressive therapy with pyrimethamine (25 to 50mg/d) and sulfadiazine (2 to 4 mg/d) because the antibiotic agents are only active against the tachyzoite stage of the protozoa (and the consequences of cyst reactivation unchecked by therapy are severe).
Intrauterine: Transplacental transmission of the organism also occurs in about one third of women who acquire T. gondii infection during pregnancy. Transmission is highest if the mother becomes newly infected during or just before (< 6 months) conception. Infections acquired >6 months before conception yields virtually no risk of transmission to the fetus, and seroconversion in the mother before pregnancy generally does not result in neonatal infection (Desmonts, 1974).
Historically, most cases of Toxoplasmosis chorioretinitis were believed to originate from congenital infection (Perkins, 1973), but recent evidence suggests that acquired toxoplasmosis may occur more frequently. Acute toxoplasmosis infection in an immunocompetent individual may be entirely asymptomatic.
Prevention: Approaches to prevention of Toxoplasma gondii infection include avoidance of oocyte contamination from feline carriers and minimizing exposure to cyst transmission from infected intermediate hosts (by cooking). Cat litter boxes and sand or dirt in outdoor areas (that may serve the same purpose for outdoor felines) should be avoided. Hand-washing after working in soil as well as careful cleaning of fruits and vegetables is recommended. Meat (especially lamb and pork) should be heated to 60 degrees centigrade for 15 minutes or frozen to kill potential cysts (Jones, 1975). Women who are pregnant or who may become pregnant as well as immunocompromised individuals should take special care in all of these precautions, especially avoiding contact with a cat litter box and other high-risk environments.
EPIDEMIOLOGY
|
SIGNS
|
SYMPTOMS
|
TREATMENT
|
Graff JM, Russell SR: Acquired Ocular Toxoplasmosis: 42-year-old female with "fuzzy" vision for two weeks. EyeRounds.org. September 14, 2007; Available from: http://www.EyeRounds.org/cases/74-Acquired-Toxoplasmosis-Retina.htm.

Ophthalmic Atlas Images by EyeRounds.org, The University of Iowa are licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License.