Corneal opacification is a primary cause of treatable blindness. Each year, approximately 100,000 corneal transplantation procedures are performed worldwide, though it is estimated that the number of patients who could benefit from the procedure is orders of magnitude greater. While the majority of transplant patients attain good visual outcomes after keratoplasty the results are often disappointing in high-risk patients (including those with a history of multiple donor graft rejections, patients with ocular surface disease, and those with limbal stem cell deficiencies). Additionally, obtaining human donor corneas is exceptionally difficult in developing countries for a variety of social, logistical and religious reasons. Because of this it is reasonable that there have been attempts to develop a substitue for human cornea tissue, keratoprosthesis.
Today, keratoprosthesis has a role in combating corneal blindness in carefully select patients with complex ocular disease who are at high risk for donor graft failure, despite the potential risk of significant postoperative complications. The most widely used keratoprosthetic devices include the Dohlman-Doane and the AlphaCor artificial corneas--the only devices approved by the United States Food and Drug Administration available for clinical use.
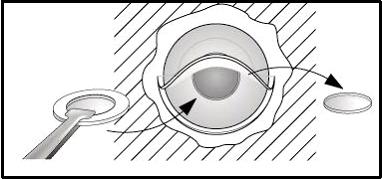
For centuries, ocular surgeons have imagined and studied a variety of methods and materials that could be used to recreate a clear, artificial window in cases of persisting corneal opacification. Some credit the French ophthalmologist Dr. Pellier de Quengsy as the first to propose a keratoprosthesis (Chirila, 1999) based on manuscripts dating as far back as 1789. Glass, polymethyl methacrylate (PMMA), various plastics, polymers, and hydrogels have been tried, with variable success. AlphaCor (Argus Biomedical Pty Ltd, Perth , Australia ) is the newest keratoprosthetic device. It was FDA-approved in August 2002 for patients at high risk for donor penetrating keratoplasty (PKP). The implant is a 7-mm diameter, one-piece, non-rigid synthetic cornea. It is composed of an outer skirt, that is an opaque, porous, high-water PHEMA (poly[2-hydroxyethyl methacrylate]), with a transparent central optic core of gel PHEMA (see Figure 1). Generally, keratoprostheses are used to treat the most complex and high-risk cases in which all other available treatment options for corneal opacification have failed. The surgery and postoperative management are not without challenges, and visual acuity is often limited by pre-existing pathology, but it appears that in many cases the AlphaCor keratoprosthesis is effective.
 |
| Reprinted with permission from Esen K. Akpek, MD10 |
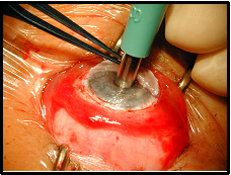
AlphaCor surgery is performed in 2 stages. In Stage I, an intrastromal trephine is used to remove the central posterior corneal lamellae for insertion of the device. A corneal incision is made and dissection instruments are used to continue the corneal dissection throughout the circumference of the corneal graft, thereby creating an intralamellar pocket (see Figure 2). An AlphaCor sizer, used to test the size and centration, is inserted into the intralamellar pocket followed by removal of the posterior disc via a 3.5 mm intrastromal trephine.
| 2A: Diagram of stage I insertion of keratoprosthesis | |
 |
|
| Reprinted with permission from Esen K. Akpek, MD10 | |
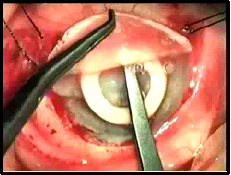
| 2B: Stage I surgery | 2C: Stage I surgery - implantation of keratoprosthesis |
 |
 |
| Reprinted with permission from Esen K. Akpek, MD10 | |
After insertion of the device and closure of the limbal incision, the surface is often covered with a Gundersen conjunctival flap. This is particularly important in cases where the ocular surface and limbal stem cell function are compromised. If the Gundersen flap is inadequate to cover the cornea an amniotic membrane graft may be required. In cases with a healthy ocular surface, a Gundersen flap may not be required, but a high oxygen permeability (high Dk/t) contact lens may suffice (Dohlman, 2002). Subconjunctival injections of antibiotics and steroid are often given in the immediate postoperative period.
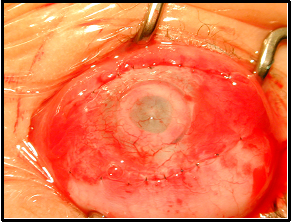 |
In Stage II, performed approximately 2 months after Stage I, the overlying conjunctiva created by the Gundersen flap is removed. This consists of trephination of the central 4mm of the conjunctival flap and anterior corneal lamellae.
| 4A: Just prior to Stage II surgery. | 4B: End of Stage II surgery. |
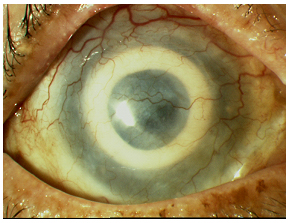 |
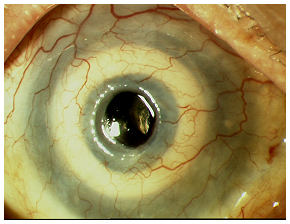 |
The initial implantation technique consisted of a superior 180° incision 1.5 mm posterior to the limbus at 50% depth and extended to form a flap of the superior cornea and a pocket within the inferior cornea to accommodate the 7.0 mm AlphaCor device. The superior flap is retracted to provide access for 3.0 mm trephination of the central posterior lamella prior to positioning the device. The procedure is completed in most cases with a Gundersen flap and/or an amniotic membrane graft to cover the corneal surface. This technique can sometimes prove lengthy, due largely to the difficulty in performing the stromal dissection. The extensive limbal disturbance and stromal dissection, both thought to further insult a generally compromised ocular surface, likely increase the risk of postoperative corneal epithelial loss and stromal melting.
Video 1 illustrates this incisional technique in a 27-year-old Caucasian male with monocular vision and sclerocornea. He has had a prior pars planar vitrectomy, seton placement, keratolimbal stem cell allograph, and two failed penetrating keratoplasties prior to AlphaCor placement.
If video fails to load, use this link: https://vimeo.com/157159207
Like the traditional large incision approach, a 50% depth incision is made 1.0–1.5 mm posterior to the limbus: however, the incision is extended for 90° instead of 180°. Subsequent lamellar dissection creates a pocket within the cornea to position the AlphaCor in the visual axis. The "sizer" is used as previously described followed by trephination through the posterior lamella and introduction of the AlphaCor. A mattress suture (10/0 nylon) is often placed through the anterior lamella to help prevent movement of the device while biointegration occurs between Stage I and Stage II. The scleral incision is closed with interrupted 10-0 nylon sutures. Lastly, the corneal surface is often covered with a Gundersen flap and/or an amniotic membrane graft in cases where the corneal surface is de-epithelialized or a high Dk/t bandage contact lens in cases with a healthy ocular surface. Stage II surgery remains as previously described in the large incision technique. The small incision technique may be particularly challenging or complication-prone in patients with very dense anterior stromal scarring that interferes the surgeon's view during trephination, when a recent graft wound might break down, and in phakic cases due to less control of entry into the anterior chamber (Crawford, 2005). Retroprosthetic membrane formation is more common using the small incision technique.
If video fails to load use this link: https://vimeo.com/157159210
In December 2004, she underwent a small incision Stage I procedure. In April 2005, Stage 2 surgery was performed, consisting of a 4 mm trephination of the central conjunctival flap and anterior corneal lamellae. Initially, postoperative best corrected Snellen acuity was counting fingers at 1 foot. By December 2005, she was fitted with a high Dk/t galyfilcon A contact lens (Acuvue Advance with hydraclear) with the following parameters: base curve (BC) = 8.30, diameter (D) = 14.0, power (P) = +6.00 sphere, Dk/t = 85. Over the course of the next four months she was able to attain a best corrected Snellen vision between 20/50 and 20/100 OS. It was thought that her visual potential was limited by entrapped air between the AlphaCor implant and the soft contact lens. She was then fitted with a piggyback system using the same Acuvue Advance contact lens OS with an overlying RGP: Transaire (silicone acrylate) dark blue 7.50 -3.00 8.6 5D reverse geometry lens which was well tolerated. The entrapped air was significantly reduced with her BCVA stabilizing between 20/40 to 20/50 OS.
This technique involves a 50% thickness lamellar dissection within the existing failed corneal graft wound and extending beyond the graft-host interface instead of beginning the dissection at the limbus. After a flap is prepared by blunt dissection through the graft-host interface, about two clock hours are left as a hinge. The flap is then reflected followed by trephination of the central 3.5 mm posterior aperture. After placement of the device the wound is sutured.
Video 3. The Stulting Technique
If video fails to load, use this link: https://vimeo.com/157159208
In terms of simplifying the procedure, the Stulting technique makes trephinaton of the posterior aperture and centration of the AlphaCor much easier, because the entire surface is exposed. Furthermore, the opening created by the trephination is more favorable to maneuvers such as manipulation of IOLs and iridotomies. Limited incision length (helpful in the presence of drainage blebs near the limbus), good exposure for trephination, and good centration of the AlphaCor device help promote better visual outcome. A possible disadvantage may be close proximity of the wound to the AlphaCor skirt which may increase the risk of corneal melt.
The possible use of IntraLase (IntraLase Corp, Irvine, CA, USA) to create a corneal pocket for the AlphaCor is intriguing. This technique may save time and may be more precise, but further investigation is warranted to examine risks and long-term visual outcomes. The femtosecond laser may be associated with less stable device placement or risk of entering the eye through irregular scarring (Nguyen, 2004).
Corneal epithelial defects begin the melting process with failure to re-epithelialize leading to either an infection or a trophic process and subsequent device failure. On the molecular level, immune mediators and collagenases attack the corneal stroma. The two most common causes of corneal melt are herpes simplex virus (HSV) keratitis and retained lenticular material. Although medroxyprogesterone (MPG) may not influence the underlying incidence of melt-related complications, which are likely to be associated with other risk factors especially HSV, it may have a protective effect with regard to melt onset and severity (Hicks, 2003; Eguchi, 2004). Although a history of HSV keratitis was a contraindication to AlphaCor in the past (Hicks, 2002), history of HSV alone is no longer considered a contraindication. In cases of corneal melt, however, HSV should be ruled out. Progressive melts are treated with lamellar graft or ‘reversed’ to a repeat attempt at PKP.
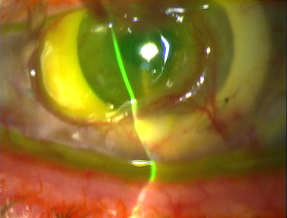 |
Retroprosthetic membranes (RPM) are a relatively common post-operative complication characterized by an inflammatory/fibrous re-closure of the posterior lamellar opening, usually composed of dense, avascular tissue. It is most strongly associated with individuals of African and Caribbean decent and patients with systemic hypertension (Hicks, 2005). Diabetes mellitus is no longer a significant risk factor, possibly reflecting successful prevention by steroids in this previously high-risk group. RPM is managed by Nd:Yag laser or surgery +/- heparin and rTPA (recombinant Tissue Plasminogen Activator). Prostaglandin inhibitors such as Latanoprost have been shown to stimulate collagen contraction mediated by human corneal fibroblasts in vitro (Yang, 2006). It remains uncertain as to the role of Latanoprost in RPM formation risk.
If video fails to load, use this link: https://vimeo.com/157159209/
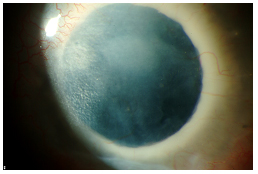
Video 4 illustrates the case of a 25-year-old Caucasian female with a history of congenital cataract, microphthalmia, and multiple seton tubes who developed significant retroprosthetic membrane following an otherwise successful keratoprosthesis surgery. She has had 3 prior PKP graft rejections with the last two rejections occurring within two weeks time. The RPM was removed surgically which resulted in a clear visual axis (see Figure 6A) other than some anterior haze (see Figure 6B).
| 6A: Slit lamp view of patient in video with red reflex showing resolution of RPM. | 6B: Some anterior haze. |
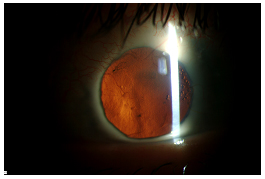 |
 |
Martel JN, Graff JM, Goins KM, Sutphin JE. The Evolution of Surgical Approaches to AlphaCor Keratoprosthesis Insertion and Associated Complications. EyeRounds.org. September 13, 2006 ; Available from: http://webeye.ophth.uiowa.edu/eyeforum/cases/60-AlphaCor-Surgical-Approaches-Artificial-Cornea-Implant.htm, 2006

Ophthalmic Atlas Images by EyeRounds.org, The University of Iowa are licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License.