Incomplete eyelid closure and ocular redness with discharge
The ophthalmology service at the University of Iowa Hospitals & Clinics was consulted for a 16-year-old male inpatient in the pediatric intensive care unit. He was admitted two months prior due to hemoptysis, anemia, and respiratory distress. After a diagnosis of idiopathic dilated cardiomyopathy was made, he underwent placement of a left ventricular assist device while awaiting a cardiac transplant. His hospital course was complicated by renal failure, sepsis, pulmonary embolism, heparin-induced thrombocytopenia, pneumothorax, oral candidiasis, and euthyroid sick syndrome. He was intubated, sedated, and mechanically ventilated throughout his hospital stay.
Bilateral incomplete eyelid closure (lagophthalmos) was noted by his primary team early during the course of his admission. He was treated intermittently with various strategies such as artificial tears on an as-needed basis, artificial tears every two hours, and erythromycin ointment three times daily. Ophthalmology was eventually consulted due to increasing ocular redness and discharge bilaterally, despite the above therapies.
The patient's mother recalled observing intermittent nocturnal lagophthalmos for years prior to his admission. He had never complained of or exhibited any ocular symptoms or signs.
Moderate developmental delay, seizures (remote)
Meropenem, vancomycin, caspofungin, midazolam, morphine, clonidine, vecuronium as needed (PRN), fentanyl PRN, lorazepam PRN, milrinone, epinephrine, argatroban, warfarin, aspirin, acyclovir, nystatin, omeprazole, levothyroxine
No family history of lagophthalmos, myopathy, or cardiomyopathy
The patient attended high school with special education assistance. He could perform his own activities of daily living prior to admission.
Visual acuity: Unable to assess due to mental status
Pupils: 2 mm (dark), 1.5 mm (bright) both eyes (OU), no relative afferent pupillary defect OU
Intraocular pressure: 15 mmHg OU
External exam (Figure 1)
Portable slit lamp exam (Figure 2)
Dilated fundus examination
Figure 1. External exam demonstrated bilateral lagophthalmos and apparent loss the Bell's phenomenon. 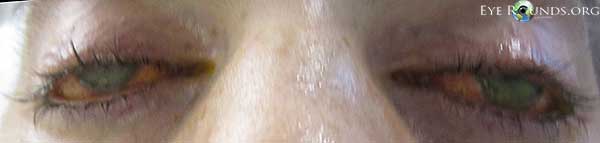
Figure 2. Slit lamp exam demonstrated blepharitis, conjunctival injection most prominent inferiorly, mucopurulent conjunctival discharge, large inferior corneal epithelial defects with a linear superior border, and corneal stromal thinning (dellen).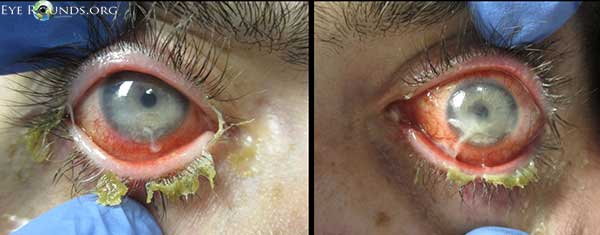
This patient demonstrated signs of bilateral exposure keratopathy due to lagophthalmos, likely related to prolonged sedation and mechanical ventilation. He also had signs of bacterial conjunctivitis in both eyes. Initially we were concerned about microbial keratitis, but after irrigation of the superficial discharge, there were no corneal infiltrates noted. Considering his mother's report of longstanding lagophthalmos prior to admission and his recent diagnosis of cardiomyopathy, we entertained the possibility of an underlying skeletal muscle abnormality that contributed to his lagophthalmos.
The inferior conjunctival fornices were swabbed and sent for Gram stain and culture. The eyelids and fornices were irrigated and cleansed thoroughly. The patient was started on topical levofloxacin solution and ophthalmic lubricating ointment to both eyes, every 6 hours. We suggested that a temporary lateral tarsorrhaphy might be needed in the future if the exposure keratopathy did not improve with conservative measures.
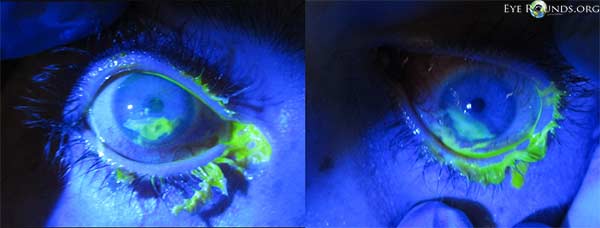
One day after initiating treatment, the blepharitis and conjunctivitis had improved considerably, but the keratopathy remained the same (Figure 3). The conjunctival gram stain revealed rare white blood cells, but no organisms.
Three days after starting treatment, there was marked improvement of the keratopathy in both eyes (Figure 4). The corneal stromal thinning of the right eye had resolved, and there was only mild staining of the inferior corneal epithelium. In the left eye, the stromal thinning and epithelial defect persisted, but both had improved considerably. The conjunctival culture revealed no growth.
Unfortunately, during the next few days, the patient's cardiovascular and renal status deteriorated, and considering his very poor prognosis, the patient's family decided to withdraw care. The patient passed away a few days later. An autopsy revealed no skeletal muscle abnormalities.
This case sparked a systems-based review of our institution's policy for prophylactic eye care for the critically ill.
Figure 3. One day after treatment with topical antibiotic solution and lubricating ointment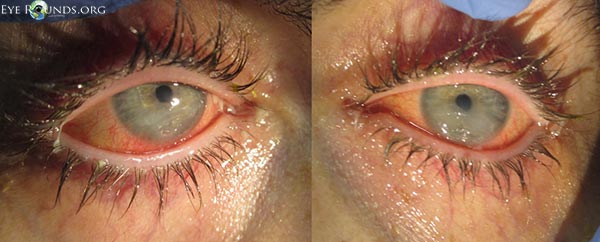
Figure 4. Three days after treatment with topical antibiotics and lubricating ointment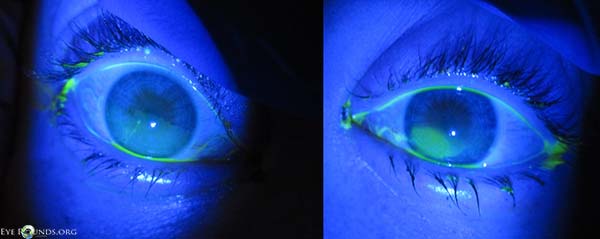
Exposure keratopathy is dryness of the cornea caused by incomplete or inadequate eyelid closure, resulting in evaporative tear loss and tear film insufficiency. Mild cases are usually benign and easily treated, but if severe, undiagnosed, and/or under-treated, drastic consequences can result. Critically ill patients are at increased risk for developing exposure keratopathy, and, because of their other serious medical issues, their corneal dryness is more likely to go undiagnosed or untreated. The purpose of this article is to briefly review the literature regarding exposure keratopathy in the critically ill and to describe how we addressed this systems-based issue at our institution.
Due to various definitions and study designs in the literature, the reported incidence of exposure keratopathy in the critically ill varies, but it can be very high. In one study of 74 ventilated intensive care unit (ICU) patients who did not receive prophylactic eye care, 57% of patients developed some form of exposure keratopathy as diagnosed by an ophthalmologist (Jammal 2012).
The major risk factor for exposure keratopathy is incomplete eyelid closure, or lagophthalmos (Jammal 2012, McHugh 2008, Merciecea 1999). Lagophthalmos may be caused by medications, chemosis, facial nerve palsy, proptosis, ectropion, trauma, or surgery. There are many factors in the critical care setting that can contribute to lagophthalmos. Neuromuscular blocking agents, which paralyze skeletal muscle, are often used in the critical care setting to improve mechanical ventilation but can impair the resting tone of the orbicularis oculi muscle which is responsible for eyelid closure during sleep (Kirwan 1997). Additionally, paralysis of the superior rectus and inferior oblique muscles may impair Bell's phenomenon, in which the globe is supraducted during eyelid closure in order to protect the cornea from exposure (Krupin 1977). Similarly, sedative medications such as benzodiazepines and propofol, which are used to decrease pain and anxiety, impair the blink reflex and result in corneal anesthesia, impairing the patient's ability to recognize symptoms (Kirwan 1997) and creating a neurotrophic component to the keratopathy. Chemosis, or conjunctival edema, also increases the risk of lagophthalmos and exposure keratopathy (Jammal 2012), and can result from increased jugular venous pressure associated with mechanical ventilation. Critical illness itself is associated with fluid retention and capillary leakage, which can also lead to chemosis (Mercieca 1999). Face mask ventilation may direct air flow to the eyes in addition to the nose and mouth resulting in increased tear evaporation. Finally, exposure keratopathy may be overlooked in the critically ill because of the many other important and life-threatening issues that must be addressed in these patients.
In critically ill patients who are often sedated or comatose, exposure keratopathy is usually asymptomatic. However, when a patient with exposure keratopathy regains consciousness, he or she might experience pain, blurry vision, and photophobia. Even if adequately treated, a patient might suffer in the future from recurrent corneal erosions. Severe cases might cause permanent corneal scarring and vision loss. The most detrimental complication is microbial keratitis, which could lead to corneal perforation, endophthalmitis, blindness, and even loss of the eye. Patients in the critical care setting may be at higher risk for these infectious sequelae because of the higher prevalence of pathogenic organisms in this environment. In one review of an intensive care unit setting, there were nine cases of bacterial keratitis in five years (Parkin 1997). In all nine cases, the organism responsible for the keratitis was also isolated from the patients' respiratory tract. Three of these patients died, but the remaining six suffered from reduced visual acuity, and four required a corneal transplant. Furthermore, when exposure keratopathy is related to intravenous sedatives and/or mechanical ventilation, it could be considered an iatrogenic condition, one that usually requires consultation with and treatment by an ophthalmologist, thus increasing the cost of care.
Since exposure keratopathy in the critically ill is common and potentially devastating, it needs to be addressed. In general, the best treatment is prevention. This is especially true for the present issue, where the cause of the problem is often iatrogenic and where critical care providers are preoccupied, as they should be, with more serious and life-threatening medical problems.
Education and awareness are frequent preventative strategies for any problem. Most critical care nurses and physicians have little, if any, formal training or experience in eye care. Theoretically, if critical care providers could recognize the signs and sequelae of exposure keratopathy, they could institute treatment on their own or consult the ophthalmology service. A realistic concern, however, is that these providers are preoccupied with other significant morbidities. In one prospective study, ICU nurses were educated about a simple grading system to assess the degree of corneal and conjunctival exposure in their unconscious and sedated patients (Suresh 2000). A treatment algorithm was then instituted and the nurses' adherence to the algorithm and the success of the prescribed treatment was monitored by an ophthalmologist. In four of the 34 patients studied, the protocol was followed incorrectly. In five of the remaining 30, nurses mis-categorized the degree of exposure. Additionally, of four patients who were supposed to be treated with taping the eyelids closed, one had their taping performed incorrectly and another had their taping discontinued without reason. Education may be helpful, but it may not be sufficient to adequately address the issue of dry eyes in critically ill patients.
Another strategy, as opposed to relying on the exam of untrained providers, is to administer prophylaxis to all patients. To be successful with universal prophylaxis, the intervention has to be effective, relatively inexpensive, and safe. In the case of exposure keratopathy, the potential strategies fall into two categories, replenishing the tear film and preventing evaporative tear loss.
Tear film replenishment can be achieved with lubricating solutions or ointments. Artificial tear solution provides necessary lubrication, but it drains easily through the nasolacrimal system and evaporates rapidly, especially in the setting of poor eyelid closure. To provide near-continuous corneal lubrication in a patient with lagophthalmos, we predict artificial tear solution would need to be administered at least hourly, likely more, which is impractical in the critical care setting for long periods of time. Furthermore, when artificial tears are needed more than four to six times a day, a preservative-free solution should be used to prevent preservative-related corneal toxicity and potential allergy, and this would increase the expense and decrease the success of this strategy.
Lubricating ointments, on the other hand, which usually consist of preservative-free petrolatum and mineral oil, last much longer because they are more resistant to drainage and evaporation. In a prospective randomized study of 50 consecutive ICU patients receiving propofol or neuromuscular blockade, lubricating ointment applied every four hours significantly reduced the incidence of exposure keratopathy compared to passive eyelid closure alone (Lenart 2000). Lubricating ointments often cause temporary blurring of vision, but this adverse effect is inconsequential in critically ill patients who are comatose or sedated.
Evaporative tear loss may be addressed by various methods such as eyelid closure or moisture chambers. Taping the eyelids closed ("tape tarsorrhaphy") is cost-effective, but in our experience this is easier said than done and can cause skin breakdown in a matter of days. A temporary tarsorrhaphy with sutures is very effective and relatively inexpensive, but this is not a realistic systemic preventative strategy and should be reserved for treatment of moderate to severe exposure keratopathy on a case-by-case basis.
A moisture chamber can also prevent evaporative tear loss and is created by using a material such as plastic wrap to cover the eye and orbit. Alternatively, goggles that seal around the eyes can be used but this increases the overall cost. Use of plastic wrap is cost-effective and has minimal adverse effects, but its practicality as a universal preventative measure is questionable and there is no evidence that a moisture chamber is effective when used without lubricating ointments. Furthermore, in a prospective randomized trial of 207 intubated, mechanically ventilated children, the addition of a moisture chamber showed no added benefit over the use of lubricating ointment every six hours (Sorce 2009).
Some have proposed the use of a polyacrylamide hydrogel dressing ("Geliperm") as a moisture chamber that not only covers the exposed globe but also assists closing the eyelid due to its adherent nature. A randomized prospective trial showed the Geliperm moisture chamber to be equally effective as lubricating ointment every six hours, but the Geliperm dressing was twice as expensive as lubricating ointment and required more training and monitoring to be effective (Ezra 2009).
After caring for the patient in this case report, we reviewed the current protocol for eye care in all 4 critical care units at the University of Iowa Hospitals and Clinics. We were encouraged to find that all 4 services had included ocular lubrication as potential orders in their frequently used electronic medical record order sets, those for admission to the unit or those for mechanical ventilation on the unit.
The orders for ocular lubrication were automatically checked in two of the four order sets, but for the others, the physician had to manually activate the order. In some cases, the order was not visible unless the physician clicked on a heading labeled "Eye Care."
One order set prompted the use of lubricating ointment alone, while the other three allowed the use of either ointment or solution. Out of the orders for solution, only one order set allowed application more than every four hours. The solution used for this order was not preservative-free.
In every instance, ocular lubricants were ordered on an as-needed basis instead of regularly scheduled intervals. Thus, the actual utilization of lubricants was dictated by nurses and whether or not they felt lubrication was necessary or even remembered to use them. We did not investigate the actual utilization of lubricants on these units, but through informal conversations, we concluded that, in general, they were used very infrequently.
After reviewing the literature, assessing the current protocols in place, and discussing the issue at our departmental Grand Rounds, we developed a new, evidence-based, systematic strategy for preventing exposure keratopathy in the critically ill at our hospital.
We felt strongly that lubricating ointment was far superior to artificial tear solution for this purpose and context. Additionally, we felt that ointment should used at regularly scheduled intervals, not on an as-needed basis. The literature suggested an interval of 4-6 hours was sufficient, so to lessen the burden in the critical care setting, we decided to go with the latter.
We liked the current strategy of including eye care orders in the standard order sets for each critical care unit, but we felt that the lubricants should be automatically ordered for every patient, not just when the ordering physician thought to use them. However, we added a clause on the order so the ointments would not be used unnecessarily in awake and alert patients.
We approached the medical directors of each critical care unit and presented our findings and recommendations. We supplied relevant literature when requested, and offered to present our case to the department if desired. Interestingly, in one instance, we were scheduled to present at a department's Morbidity and Mortality Conference, but there was never time for our presentation because there were already so many other serious issues to discuss.
Our proposal was accepted in every unit and the following standing order was added to each of the standard admission order sets for the critical care units at the University of Iowa Hospitals & Clinics:
Ocular lubricant ointment every 6 hours to both eyes, while the patient is sedated and/or ventilated
We believe this simple, strategic, evidence-based intervention will provide the best prophylactic eye care for our critically ill patients.
Ezra DG, Chan MP, Solebo L, Malik AP, Crane E, Coombes A, Healy M. Randomised trial comparing ocular lubricants and polyacrylamide hydrogel dressings in the prevention of exposure keratopathy in the critically ill. Intensive Care Med. 2009 Mar;35(3):455-61. [PMID: 18810388]
Jammal H, Khader Y, Shihadeh W, Ababneh L, Aljizawi G, AlQasem A. Exposure keratopathy in sedated and ventilated patients. J Crit Care. 2012 Dec;27(6):537-41. [PMID:22516144]
Kirwan JF, Potamitis T, el-Kasaby H, Hope-Ross MW, Sutton GA. Microbial keratitis in intensive care. BMJ. 1997 Feb 8;314(7078):433-4. [PMID: 9040392]
Krupin T, Cross DA, Becker B. Decreased basal tear production associated with general anesthesia. Arch Ophthalmol. 1977 Jan;95(1):107-8. [PMID: 836195]
Lenart SB, Garrity JA. Eye care for patients receiving neuromuscular blocking agents or propofol during mechanical ventilation. Am J Crit Care. 2000 May;9(3):188-91. [PMID: 10800604]
McHugh J, Alexander P, Kalhoro A, Ionides A. Screening for ocular surface disease in the intensive care unit. Eye (Lond). 2008 Dec;22(12):1465-8. [PMID: 17721505]
Mercieca F, Suresh P, Morton A, Tullo A. Ocular surface disease in intensive care unit patients. Eye (Lond). 1999 Apr;13 ( Pt 2):231-6. [PMID: 10450388]
Parkin B, Turner A, Moore E, Cook S. Bacterial keratitis in the critically ill. Br J Ophthalmol. 1997 Dec;81(12):1060-3. [PMID: 9497465]
Sorce LR, Hamilton SM, Gauvreau K, Mets MB, Hunter DG, Rahmani B, Wu C, Curley MA. Preventing corneal abrasions in critically ill children receiving neuromuscular blockade: a randomized, controlled trial. Pediatr Crit Care Med. 2009 Mar;10(2):171-5. [PMID: 19188877]
Suresh P, Mercieca F, Morton A, Tullo AB. Eye care for the critically ill. Intensive Care Med. 2000 Feb;26(2):162-6. [PMID: 10784303]
Risma JM, Syed NA. Exposure Keratopathy in the Critically Ill: A Case Report, Discussion, and Systems-Based Intervention. EyeRounds.org. July 1, 2014; available from https://eyerounds.org/cases/189-exposure-keratopathy.htm<

Ophthalmic Atlas Images by EyeRounds.org, The University of Iowa are licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License.