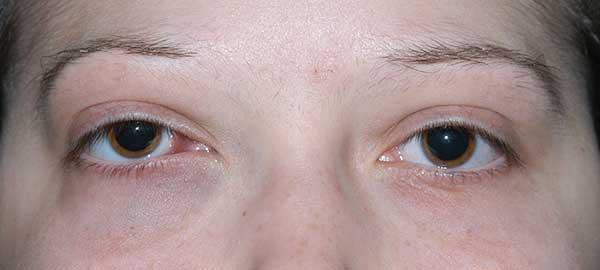
Chief Complaint: Sensation of fullness of the right eyelid and double vision.
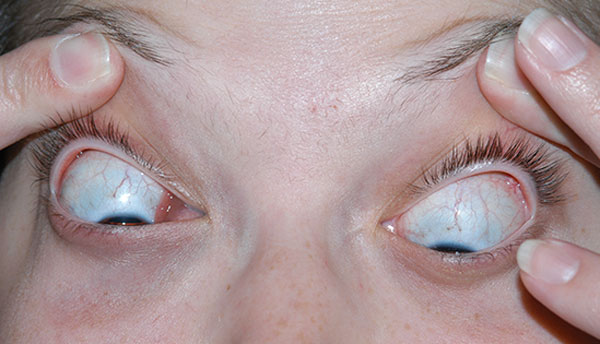
History of Present Illness: A 20-year-old female was referred to the University of Iowa Hospitals and Clinics (UIHC) Oculoplastics Service by her oncologist for a two-week history of intermittent binocular diplopia that worsened with right head tilt. The patient also noted a six-week history of progressive fullness of the right upper eyelid.
Past Ocular History: Significant for mild myopia and a history of corneal ulcers secondary to contact lens use. No prior trauma or surgeries.
Past Medical History: Acute Myelogenous Leukemia (FLT3-mutated, trisomy 8) reportedly in remission. Patient is now 1 year status-post chemotherapy and allogeneic bone marrow transplant. Other Medical History includes sinusitis, migraines, pancreatitis, allergic rhinitis, and gastroesophageal reflux.
Prior Chemotherapy: Induction therapy with cytarabine and daunorubicin. Consolidation therapy with high dose cytarabine. Preconditioning therapy prior to bone marrow transplant with busulfan and cytoxan.
Family History: Grandmother with breast cancer and cataracts.
Social History: Denies alcohol or tobacco use. Currently on medical leave from college.
Review of Systems: Otherwise negative.
 |
 |
Given this patient's history of acute myeloid leukemia (AML), there was concern that this mass was an extramedullary manifestation of her leukemia. An orbital ultrasound (see Figure 2) was performed which revealed an oval to lobular irregular mass measuring 18.35mm in height by 13.48mm at the base located in the right lower lid.
|
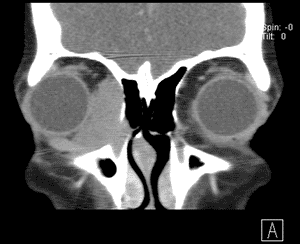
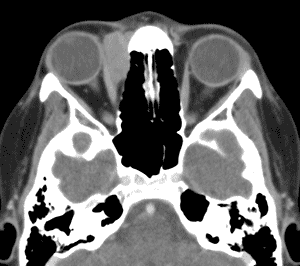
An orbital CT (see Figure 3) revealed a homogenously enhancing extraconal mass measuring 2.0 x 2.8 x 2.7cm located medially in the right orbit adjacent to the medial rectus muscle and coursing within the lacrimal duct. The mass molded to the globe and appeared to cause some lateral displacement which was likely responsible for her diplopia.
 |
 |
The patient underwent a right anterior orbitotomy with incisional biopsy. Dissection revealed a greenish mass at the caruncle that was infiltrating the nasal fat pad. The biopsy was sent to F.C. Blodi Eye Pathology at the University of Iowa. On gross inspection, the mass appeared as multiple pieces of firm, irregular, tan tissue on filter paper in formalin with an aggregate measurement of 13 x 7 x 3mm.
Histologic examination demonstrated fragments of soft tissue with significant crush artifact. The intact cells were poorly differentiated with pleomorphic nuclei often demonstrating a vacuolated, stippled staining pattern, prominent nucleoli, and scant cytoplasm (see Figure 4a and b).
Immunohistochemistry was performed (see Figure 4c and d). Nearly half of the cells stained positive for CD45. Myeloperoxidase (MPO) was positive in nearly all cells. CD117 was equivocal for technical reasons. Only the vascular endothelial cells of blood vessels were positive with CD34. Flow cytometry determined that of the viable population of cells in the sample, 7% were CD45 positive and 63% were positive for CD117 and myeloid antigens (CD13, CD33). These findings showed 3.8% myeloid blasts. These findings are consistent with a diagnosis of extramedullary acute myeloid leukemia (myeloid sarcoma).
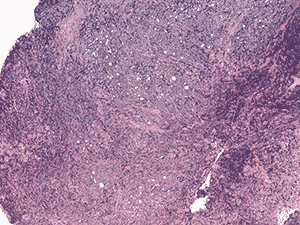
4a. 20X H&E |
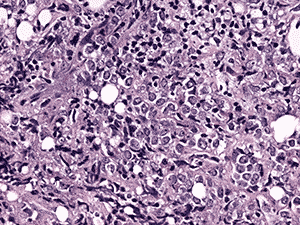
4b. 200X H&E |
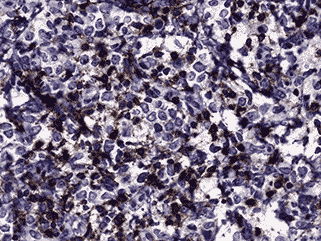
4c. 200X CD45 |
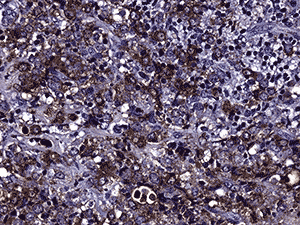
4d. 200X MPO |
A bone marrow biopsy and lumbar puncture were performed and revealed no evidence of CNS leukemia or hematologic relapse. The patient then received 12 mg of intrathecal methotrexate for CNS prophylaxis and was scheduled for close follow-up with hematology oncology.
The patient was seen by radiation oncology and scheduled to receive local radiation therapy at a dose of 30 Gy in 15 fractions for the treatment of her orbital myeloid sarcoma.
The patient was seen one week post-biopsy in the ophthalmology clinic just prior to starting radiation therapy. The patient denied any pain and noted decreased diplopia with some persistence of symptoms on left gaze with a right adduction deficit.
The patient was last seen by hematology after her third dose of radiation. She noted a dramatic reduction in the size of her orbital mass and improvement in her symptoms with rare diplopia. She has noticed some increased dryness in that eye and uses artificial tears as needed.
Final Diagnosis: Extramedullary acute myeloid leukemia (myeloid sarcoma).
Ophthalmic involvement is a well-documented complication of acute leukemia. Retinal lesions, as first described by Liebreich in the 1860s, are among the most common clinical ocular manifestations of leukemia; however, leukemia may present through a variety of ocular, orbital, adnexal, or neuro-ophthalmic manifestations.
Ocular structures become involved via two distinct mechanisms. Primary involvement is characterized by direct infiltration of leukemic cells and may occur as anterior segment uveal infiltration, orbital infiltration, or central nervous system (CNS) involvement with neuro-ophthalmic signs. Secondary ocular involvement is due to the hematologic abnormalities seen in leukemia. Anemia and thrombocytopenia can produce tissue ischemia and hemorrhage, while leukostasis and immunosuppression may predispose to occlusion injury and infection, respectively. In a prospective study of 120 patients newly diagnosed with leukemia, 3% had primary ocular involvement, 39% showed secondary manifestations, and 5% had visual loss. The rate of visual loss reported in this study is likely an underestimate as many of these patients were either too sick or too young to undergo visual acuity testing (Schachat, 1989).
Ophthalmic involvement is most commonly encountered in the setting of acute myelogenous leukemia, but may also be seen in other myeloproliferative and myelodysplastic disorders. Over 80% of eyes at autopsy show some evidence of leukemic infiltration, with the choroid being the most commonly involved site on histology (Schachat, 1989). Ophthalmic findings may precede the diagnosis of leukemia, occur concurrently with systemic disease during blast crisis, or present as the first sign of relapse, with or without bone marrow involvement. This presents a diagnostic challenge for patients with no prior history of leukemia and illustrates the importance of ophthalmic evaluation at the time of diagnosis.
Myeloid sarcoma is an extramedullary tumor of immature myeloid cells. Myeloid sarcomas were initially termed ‘chloromas,’ due to their green appearance on gross examination. This appearance is due to the presence of myeloperoxidase enzymes in the immature myeloid cells. These tumors have also been referred to as granulocytic sarcomas; however, as not all leukemias are of the granulocytic cell type, myeloid sarcoma is the preferred nomenclature.
Leukemic cells are known to exhibit a predilection for bone and periosteum as these are sites of active hematopoiesis. It is thought that leukemic cells travel through the haversian canals to infiltrate the periosteum, which then serves as the origin for subsequent involvement of adjacent orbital structures. Myeloid sarcoma is most commonly found in the orbit and subcutaneous soft tissues but may also present in the lacrimal gland, iris, conjunctiva, sclera, lymph nodes, skin, and kidney. It is most commonly associated with AML types M2, M3, M5a, and M5b and has been associated with various chromosomal abnormalities including t(8;21) and inv(16).
Myeloid sarcoma is more common in children, with a median age of 7 years and is present in 2.5-9.1% of patients with AML (Bakst 2011). Myeloid sarcoma occurs with a slight male predominance and is more commonly reported in the Middle East, Asia, and Africa.
The clinical presentation of myeloid sarcoma is dependent on the site of involvement. In a review of reported cases, Shields estimates that proptosis is the first sign at presentation in 86% of cases with over 60% presenting bilaterally (Shields, 2003). Proptosis is often slowly progressive and may occur at any point in the disease process. A review of 33 cases demonstrated that clinical presentation preceded systemic leukemia in 88% of cases (Zimmerman, 1975). This study also demonstrated that untreated isolated myeloid sarcoma will inevitably progress to AML or hematologic relapse within a median time of 5 to 12 months from the time of presentation.
Diagnosis of myeloid sarcoma requires biopsy with histologic and immunohistochemical evaluation. Histology demonstrates a diffuse monotonous infiltrate with cytologic variability. Cells are often poorly differentiated with pleomorphic nuclei and prominent nucleoli. Immunohistochemistry is performed to confirm cellular origin and arrive at a definitive diagnosis. Myeloperoxidase and lysozyme stains are the best stains to clench the diagnosis of myeloid sarcoma; however, these cells often stain positive for a host of other markers. In this case, cells stained positive for CD45, a hematopoietic stem cell marker, and myeloperoxidase, a marker for the myeloid lineage. A study of 30 cases showed CD117 reactivity in 87%, MPO, 97%; lysozyme, 93%; CD34, 47%; CD45, 84%; CD43, 97%; TdT, 37%; CD79a, 20%; CD20, 10%; CD3, 10%; and CD10, 1% (Chen, 2001).
Myeloid sarcoma is associated with a poorer prognosis than AML alone, lower rates of complete remission, and a 5-year survival estimate of 20-30% (Bakst, 2011). There are currently no standard criteria for the treatment of these patients and there are few studies on post-remission chemotherapy for isolated myeloid sarcoma. However, the treatment approach should target the underlying malignancy. Patients are generally managed on an individual basis and may undergo local radiation therapy with systemic and/or intrathecal chemotherapy.
|
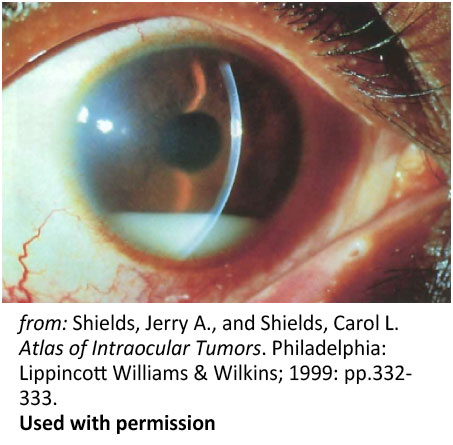 Figure 6: Pseudohypopyon. Spontaneous pseudohypopyon in a 23-year-old patient with acute lymphoblastic leukemia |
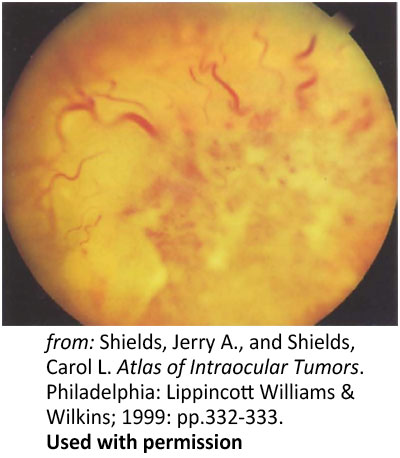 Figure 7: Primary infiltration of the optic nerve. Optic nerve head infiltration in a 28-year-old with leukemia |
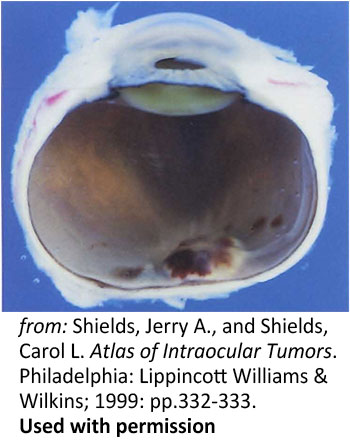 Figure 8: Disc hemorrhage. A photograph of a a sectioned eye obtained post-mortem showing hemorrhagic swelling of the optic disc and surrounding tissues |
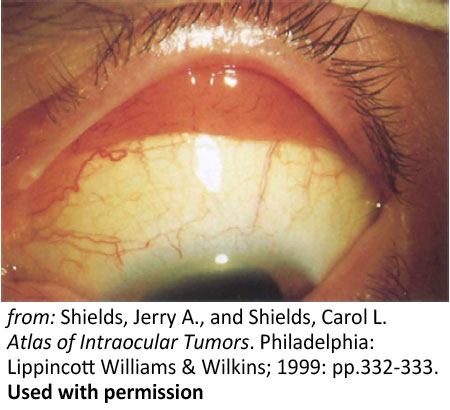 Figure 9: Conjunctival lesions. Salmon-colored leukemic infiltration of the superior fornix |
In summary, ophthalmic manifestations are not uncommon among patients with acute leukemia or other myeloproliferative disorders. Patients may present with a range of orbital, ocular, or neuro-ophthalmic manifestations. Presentation may precede systemic leukemia, occur during blast crisis, or be the first sign of relapse, with or without bone marrow involvement. This illustrates the importance of ophthalmic evaluation at the time of diagnosis. There are no standard criteria for treatment; however, management should target the underlying malignancy and require close communication between the ophthalmologist, pathologist, and oncologist.
Warren CC, Risma J, Allen RC, Syed NA. Myeloid Sarcoma: a 20-year-old female with lid fullness and diplopia. EyeRounds.org. December 6, 2011. Available from: https://eyerounds.org/cases/141-Myeloid-Sarcoma.htm.

Ophthalmic Atlas Images by EyeRounds.org, The University of Iowa are licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License.