
INITIAL PRESENTATION
Chief Complaint: Drooping of the left upper lid.
History of Present Illness:
A 7 month-old male patient was referred to the Oculoplastics Clinic at the University of Iowa by his pediatric ophthalmologist for evaluation of left upper eyelid ptosis. The mother stated that the eyelid had been "droopy" since birth and that it appeared to be getting progressively worse. She denied any alteration of eyelid position with feeding. He had been followed closely by his pediatric ophthalmologist and had previously shown no signs of amblyopia of the left eye. However, at his most recent visit he appeared to be favoring his right eye, which prompted the referral to the Oculoplastics service. The mother had also been advised to begin part time occlusion of the right eye to address the developing amblyopia.
Medical History:
The patient was born full term after an uncomplicated pregnancy and delivery. There was no history of birth trauma. He had reached all of his developmental milestones.
Medications: None
Allergies: None
Family History: : There was no history of congenital ptosis in the family.
Social History: The patient lives and is cared for by both parents.
EXAM
Visual Acuity (without correction): Fixes and follows OD, Fixes and follows OS. He had demonstrated a fixation preference for the right eye at his recent pediatric ophthalmology exam.
Pupils: No anisocoria and no relative afferent papillary defect.
Motility: Full OU.
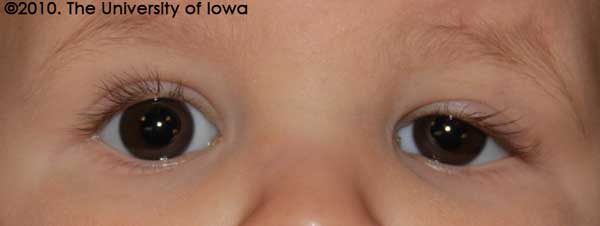
External Exam: Ptosis of the left upper eyelid. There was no variability of eyelid position with feeding or in different gaze positions. There were no palpable masses in the upper eyelid (see Figure 1).
External Measurements:
| Right | Left | |
|---|---|---|
| Palpebral Fissure | 9 mm | 5 mm |
| MRD1 | 4 mm | 0 mm |
| Levator Function | Normal excursion and velocity | Poor excursion |
Anterior segment exam: Normal conjunctiva, corneas, anterior chambers, irides and lenses OU.
Dilated funduscopic exam: Normal macula, vasculature and periphery OU.
CLINICAL COURSE
This patient's findings were consistent with unilateral, isolated congenital ptosis. The patient had no other ocular or systemic findings associated with the ptosis, and he had no palpable masses in the upper eyelid that could cause a secondary ptosis.
Because the patient had begun to develop a fixation preference for the right eye, the decision was made to intervene surgically to prevent the development of amblyopia. Given the poor levator function on the left side, a Supramid sling procedure was chosen. Consent was obtained from the mother, and the patient underwent an uncomplicated Supramid sling procedure on the left side shortly thereafter.
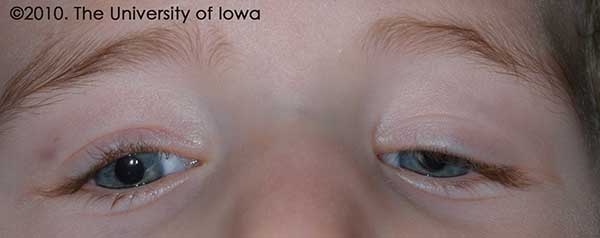
The patient returned for a post-operative visit one week later and then at 4 months after the surgery. At his four month follow up visit, his lid position was noted to be improved and his eyelid margin was above the visual axis. (See Figure 2.) He had been seen by his pediatric ophthalmologist in the interim and was noted to have equal fixation with both eyes; his patching regimen was therefore discontinued. While there was still some difference in eyelid position between the two sides, the goal of reversing his amblyopia had been achieved. The mother was advised that further surgery may be necessary in the future to address any eyelid asymmetry.
DIAGNOSIS: Diagnosis: Isolated congenital ptosis
Differential Diagnosis
DISCUSSION
Congenital ptosis can be separated into ptosis that occurs in isolation (simple ptosis) and ptosis that occurs in association with other ocular findings or systemic conditions. Understanding the etiology of the ptosis is imperative for accurate surgical planning and in the management of a patient's underlying systemic condition. Here we will discuss the various types of ptosis and follow this with a discussion of the various treatment options.
As demonstrated in this case, coordinating care with a pediatric ophthalmologist is essential to the management of these patients. Every patient being evaluated for congenital ptosis requires a complete ophthalmic evaluation with special attention to amblyopia, anisometropia and irregular astigmatism induced by the ptotic eyelid. These findings often dictate surgical management as will be discussed below.
One of the most important elements of the examination of a patient with apparent congenital ptosis is to determine whether the patient's eyelid is actually primarily ptotic or ptotic secondary to another physical process. Subcutaneous masses like plexiform neurofibromas, capillary hemangiomas and lymphangiomas can all masquerade as unilateral ptosis and should not be confused for true ptosis.
Congenital Ptosis
Isolated Congenital Ptosis
The majority of congenital ptosis cases represent an isolated eyelid malposition, absent other ocular or systemic associations. The ptosis can be unilateral or bilateral and is typically noticed shortly after birth. It is generally considered to be a non-progressive condition with persistent ptosis that is not altered with eye movement or innervation of other cranial nerves. Sometimes a familial element is present, but a gene responsible for bilateral congenital ptosis has yet to be identified.
The unifying clinical feature of isolated congenital ptosis is poor levator function. The etiology of this dysfunction is not clear, and there has been debate as to whether this represents a primary muscular or primary neurologic problem. There is some evidence that supports a neurological explanation, namely that the failure to properly innervate the developing levator palpebrae results in poor development of the muscle (McMullan 2006). The outcome of this developmental insult is the creation of a dysgenic levator palpebrae muscle in which the normal striated muscle fibers are replaced with fatty or fibrotic tissue, especially in the anterior portion of the muscle (Baldwin 2002). The dysgenic muscle can neither contract nor relax normally: The clinical consequence is poor eyelid elevation on upgaze and eyelid lag on downgaze.
While most patients with isolated congenital ptosis have no ocular motility deficits, some patients have associated ipsilateral superior rectus weakness (Beard 1976). This is thought to be secondary to a combined dysgenesis of the levator/rectus complex, also as a result of a developmental insult.
Ptosis Associated with Ocular and Systemic Abnormalities:
Blepharophimosis-Ptosis-Epicanthus Inversus Syndrome (BPES)
First described by Komoto in 1921, blepharophimosis-ptosis-epicanthus inversus syndrome (BPES) is a dominantly inherited disorder characterized by four features that are present at birth. Patients have:
The severity of the ptosis and blepharophimosis require most children to adopt a chin-up backwards head-tilt position and to recruit the frontalis in elevating the lids, leading to raised, arched eyebrows. BPES is categorized into two types: Type I is characterized by the abovementioned eyelid findings and premature ovarian failure and infertility. Type II is characterized by eyelid findings without ovarian failure. Other ocular characteristics that have been reported in association with BPES include euryblepharon, strabismus, microphthalmos, lacrimal drainage abnormalities and optic disc coloboma. Extraocular manifestations include a broad, flat nasal bridge, arched palate, and cup-shaped ears (Allen 2008).
BPES is associated with a dominantly inherited mutation in the FOXL2 gene on chromosome 3q23. This gene is expressed primarily in the developing eyelid and in the ovary. Up to 75% of patients with BPES have relatives who have the FOXL2 mutation; the remaining 25% of cases represent either new mutations or milder expression in prior generations (Allen 2008). Type I BPES is characterized by complete penetrance and transmission almost exclusively through males (because of impaired female fertility). Type II BPES is characterized by incomplete penetrance and transmission by both males and females.
The treatment of blepharophimosis requires coordination among oculoplastic surgeons, pediatric ophthalmologists, pediatric endocrinologists and genetic counselors. The surgical repair of the eyelid is complex because of the numerous and interdependent eyelid findings. The repair of ptosis is usually addressed first with frontalis suspension (described later). Early surgery for ptosis is advised if the ptosis is severe and amblyogenic. The canthal repair is usually addressed after ptosis, though some advocate canthal repair first. Medial canthoplasty can be accomplished by a combination of flaps and, at times, transnasal wiring. Some also suggest fixing the medial canthus to the periosteum.
Marcus-Gunn Jaw Winking
Marcus-Gunn jaw-winking ptosis was first described in 1883 as unilateral upper eyelid ptosis with eyelid retraction associated with activation of the pterygoid muscle (i.e. movement of the jaw) (see Figures 4 and 5). This is a congenital condition thought to be caused by pterygoid-levator synkinesis. The exact abnormal neurological pathway has yet to be described, but it is postulated that fibers of the fifth cranial nerve are responsible for either directly or indirectly innervating the levator. Electromyographic studies have reported that the impulses generating levator activation originate from the proprioceptive receptors of the pterygoid muscles, thereby linking pterygoid movement to elevation of the eyelid. The condition is almost always unilateral and affects males and females in equal proportion (Demirci 2010). There is associated superior rectus weakness in over half of cases (Beard 1976).
In evaluating a patient with what appears to be isolated unilateral ptosis, it is valuable to ask if the infant's ptosis varies with nursing or eating. This may be a clue to look carefully for this synkinetic condition.
Figures 4 and 5: This is a patient with Marcus-Gunn Jaw Winking ptosis. (Click image for higher resolution)
Congenital fibrosis of the extraocular muscles (CFEOM)
Congenital fibrosis of the extraocular muscles (CFEOM) is a rare, non-progressive condition that results in restrictive global ophthalmoplegia and congenital ptosis. While CFEOM was classically thought to be a myopathy that resulted in muscle fibrosis, there is recent evidence to suggest that the fibrotic changes are secondary to defective innervations of the muscles during development (Heidary 2008). The eyes are most often fixed in an infraducted position, approximately 20-30 degrees below the horizontal midline. This downward gaze, combined with the ptosis, necessitates a chin-up position in many patients (Heidary 2008) (see Figure 6).
Four phenotypes have been described: CFEOM 1, 2, 3 and Tukel syndrome (CFEOM 3 phenotype with postaxial oligodactyly or oligosyndactyly). CFEOM 1 and 3 are inherited in an autosomal dominant fashion and are most commonly associated with missense mutations in the KIF21A gene. While CFEOM 1 typically involves bilateral ptosis and ophthalmoplegia, CFEOM 3 has a more variable phenotype in which ptosis may be unilateral, ophthalmoplegia may be mild and some family members may be unaffected. Mutations in the PHOX2A gene have been associated with CFEOM 2; these are inherited in an autosomal recessive fashion.
These patients require a stepwise surgical approach to correct strabismus and eyelid position. The vertical and horizontal misalignments are addressed first followed by the ptosis repair, as extraocular muscle surgery can alter eyelid position.
Congenital Myasthenic Syndromes (CMS)
Congenital myasthenic syndromes are a heterogeneous group of syndromes characterized by defects in neuromuscular transmission. These patients present in infancy or early childhood with fatiguable ocular and/or extremity weakness. Ocular manifestations include ptosis, orbicularis weakness and strabismic deviations.
These disorders are distinguishable from myasthenia gravis (MG) in the etiology of the transmission defect; while the transmission defect in MG is caused by antibodies against the acetylcholine receptor, CMS is caused by genetic presynaptic, synaptic, or postsynaptic defects at the neuromuscular junction. The inheritance of these syndromes can be autosomal dominant or recessive. Initial therapy is usually medical and tailored to the exact neuromuscular deficiency. Surgical correction of ptosis is reserved for those cases that do not respond to medical therapy (Sieb 2002).
Chronic Progressive External Ophthalmoplegia (CPEO)
Chronic Progressive External Ophthalmoplegia (CPEO) is a mitochondrially inherited disorder that is characterized by ptosis and ophthalmoplegia secondary to progressive weakness of the extraocular muscles. There is often also associated orbicularis weakness. While the mean age of diagnosis is generally in the fourth or fifth decade, the disease can manifest in infancy and childhood as well. The majority of patients present with single deletions in the mitochondrial DNA, and transmission is maternal.
The clinical features of this disorder are ptosis and progressive ophthalmoplegia. In later stages, the eyes are eventually fixed in primary gaze, or slightly infraducted. These clinical features are ascribed to a myopathy selectively affecting the extraocular muscles and levator complex; biopsy of the affected muscles typically demonstrates ragged red fibers and atrophy of selected muscle fibers.
Kearns-Sayre syndrome is characterized by the clinical features of CPEO combined with pigmentary retinopathy, complete heart block and cerebellar ataxia. Patients with CPEO must be evaluated for this potentially fatal syndrome and treated accordingly.
Treatment of the ptosis in this condition is primarily surgical. The use of coenzyme Q has been reported but has not been shown to be effective in treating this condition (Caballero 2007).
Please see our dedicated CPEO EyeRounds case for a more extensive discussion of this condition.
Congenital Third Nerve Palsy
Third nerve palsies that are present at birth can be due either to a developmental abnormality or intrauterine/birth trauma. They represent nearly half of third-nerve palsies seen in children. Infants usually present with unilateral ptosis, some amount of ophthalmoplegia and pupil involvement (either dilated or miotic because of aberrant regeneration). The frequency of aberrant innervation seen in these cases suggests that the majority of the lesions occur along the peripheral nerve; however, central lesions have also been reported.
An uncommon association with congenital third nerve palsies is cyclic oculomotor paresis. Thought to be a form of aberrant innervation, cyclic third nerve palsies present as baseline oculomotor paresis with episodic third nerve activation resulting in miosis, elevation of the ptotic upper eyelid and adduction of the eye. These cycles can be debilitating as they often occur frequently and rhythmically. Noting this aberration prior to surgical planning is important, as correcting ptosis in such a patient will not change the cyclic changes of eyelid position (Biousse 2000).
Congenital Horner's Syndrome
Horner's syndrome results from a defect in the sympathetic innervation to the eye and adnexal structures and causes an ipsilateral ptosis, miosis of the pupil and anhydrosis of the affected side of the face. The ptosis associated with Horner's syndrome is unique in that it results from Mueller's muscle inactivation as opposed to any sort of levator dysfunction.
Horner's syndrome is most commonly seen as an acquired condition in adults; less than 5% of cases can be classified as truly congenital. The most common cause of congenital Horner's syndrome is birth trauma resulting in a brachial plexus injury. Potentially life-threatening causes of congenital Horner's, including thoracic and cervical neuroblastoma, agenesis of the internal carotid artery and complications from perinatal surgical procedures have also been reported. Although less common, congenital Horner's resulting from carotid artery aneurysms and traumatic carotid dissection have also been described (Mirzai, 2006).
Ptosis secondary to a Horner's syndrome can usually be distinguished from isolated congenital ptosis because of the associated ocular and adnexal findings. Infants present with unilateral ptosis but also with associated miosis. Parents may report that the affected side of the face does not flush when the infant cries (so-called Harlequin baby'). In cases of congenital Horner's associated with sympathetic dysgenesis (and not birth trauma) the affected iris may be lighter, as iris melanocytes, like sympathetic ganglion cells, derive from neural crest cells.
Treatment of Ptosis
When to treat
The primary indications for treatment of any form of congenital ptosis are amblyopia and abnormal head positioning. In the setting of severe unilateral ptosis, the ptotic lid position can be very amblyogenic, and early surgery is advised. Severe bilateral (or unilateral) ptosis can cause a patient to assume an obvious chin-up head position that can interfere with the child's normal functioning. If neither of these two issues is at play, the correction of ptosis can be delayed until the patient is old enough to have the surgery performed with only local anesthesia, as this makes intraoperative adjustments possible.
If a patient with ptosis also requires strabismus surgery, operating on the vertical muscles can alter the eyelid position. Therefore, ptosis surgery is often deferred until after the strabismus has been corrected.
How to treat
Levator Resection
The levator resection surgery is an intervention used in those patients who have some amount of levator function. Unlike a levator aponeurosis advancement in adults, the amount of advancement cannot be adjusted intraoperatively (assuming the patient is a child and the procedure is being done under general anesthesia); the amount of resection is decided preoperatively. Another difference between adult and pediatric levator resections is that children with congenital ptosis generally require a much larger levator advancement. Most levator resections in children require a dissection superior to Whitnall's ligament except in the mildest of cases, but this is rarely necessary when correcting adult involutional ptosis.
There are numerous ways to estimate the amount of levator resection necessary to correct congenital ptosis. The two methods that are most commonly cited and used are those explained by Beard and Berke in their respective reports of congenital ptosis repair.
The method explained by Beard incorporates both eyelid excursion and amount of ptosis to estimate the amount of levator to be resected. Please see Table 1 for the specific figures for this estimation.
| Amount of Ptosis | Upper eyelid excursion | Amount of Resection |
|---|---|---|
| 0-5 mm (poor) | 22-27 mm | |
| 2 mm (mild) | 6-11 mm (fair) | 16-21 mm |
| 12 or more (good) | 10-15 mm | |
| 0-5 mm (poor) | Maximum (30 mm) | |
| 3 mm (moderate) | 6-11 mm (fair) | 22-27 mm |
| 12 or more (good) | 16-21 mm | |
| 0-5 mm (poor) | Maximum (30 mm) | |
| 4 mm or more (severe) | 6-11 mm (fair) | 25-30 mm |
| 12 or more (good) | 25-30 mm |
The method described by Berke uses upper eyelid excursion not to determine the amount of levator that needs to be resected, but rather to determine the intraoperative eyelid height at the end of the surgery. The idea behind this approach is that an eyelid with good excursion will rise from its final intraoperative height, and an eyelid with poor excursion may drop from its final intraoperative position. Table 2 details this approach:
| Upper eyelid excursion | Superior corneal coverage by upper eyelid |
|---|---|
| 0-5 mm (poor) | 0 mm (lid margin at superior limbus) |
| 6-11 (fair) | 2 mm |
| 12 or more (good) | 4 mm |
These two tables represent not concrete rules, but rather guidelines to assist in surgical planning for congenital ptosis. Most oculoplastic surgeons probably use some combination of these two methods and adjust their operative approach on a case-by-case basis.
Frontalis Sling
This is the most common surgery performed for congenital ptosis and is especially useful in instances where the levator function is very poor. In this procedure, the eyelid is coupled to the frontalis muscle using either a fascial sling or a sling made of a synthetic material (discussed below). By recruiting the frontalis muscle to raise the eyelids, this procedure relieves the dysfunctional levator from contributing to eyelid elevation.
There are a number of materials that can be used to sling the frontalis to the eyelid; each has its own indications, advantages and disadvantages.
Sling materials:
Fascia lata: Fascia lata, the deep fascia of the thigh, is one of the most commonly used materials for frontalis sling. Fascia lata can either be harvested from the patient (autogenous fascia lata) or be provided by a tissue bank (allogenic fascia lata)
Autogenous: Autogenous fascia lata is thought to be the gold standard material for a frontalis sling. It has excellent tensile strength and, as a living tissue, biointegrates into the eyelid/forehead environment in a way that makes rejection rare and absorption of the material/recurrence of ptosis equally unlikely. One disadvantage of autogenous fascia lata is the creation of a second donor site and the infectious risks therein. Also, it is generally difficult to harvest enough fascia from a child under the age of three, so autogenous fascia is not an option for infants.
Allogeneic: Allogeneic fascia has all of the advantages of autogenous fascia and can be used in infants under the age of three. A second donor site is not required in these cases. There are the theoretical risks of rejection of the sling material and the transmission of infection from the donor to recipient, but generally speaking these slings are well tolerated.
Supramid:
Supramid Extra, a 4-0 polyfilament cable-type ophthalmic suture, is a synthetic alternative to fascia lata. Its advantage is that it does not require a donor site and can be used in children of any age. Supramid has, however, been associated with recurrences of ptosis and is not thought to be a reliable permanent solution for congenital ptosis (Katowitz 1979). Scanning electron micrographs of Supramid used in ptosis cases that recurred show that the material undergoes a morphologic degradation by hydrolysis, supporting its use as a temporary measure only (Kook 2004). Supramid is generally reserved for infants/children who need their ptosis addressed quickly (because of amblyopia, for example) but who will later undergo a more permanent sling.
Silicone:
Silicone is another synthetic alternative to fascia that also does not require a donor site and can be used in patients of any age. Unlike Supramid, silicone is not associated with frequent recurrence of ptosis and is often used as a permanent solution for congenital ptosis (Lee 2009). The other advantage is that the material is elastic and can be adjusted, both intraoperatively and post-operatively, if the eyelid height needs to be altered over time.
Controversies in Treatment
There are a number of unique clinical scenarios for which treatment is controversial. Two such scenarios are described below.
Unilateral ptosis with poor levator function:
Unilateral sling: Recruiting the ipsilateral frontalis muscle using a unilateral frontalis sling is an option for unilateral ptosis and poor levator function. This is an attractive option because it does not rely on the poorly functioning levator and because it does not require surgery on the unaffected eyelid. One of the concerns with a unilateral sling is asymmetry between the lids especially on downgaze, with pronounced lagophthalmos on the operative side. Another concern is whether patients will have spontaneous unilateral frontalis action to utilize the sling. This may not be a problem for non-amblyopic patients, but patients who are amblyopic on the operative side or have a fixation preference may have variable success with the unilateral sling (Kersten 2005).
Bilateral sling with unilateral disinsertion of levator: To overcome the potential asymmetry associated with a unilateral sling, Beard proposed disinserting the levator on the unaffected side (causing an iatrogenic severe ptosis) and performing a bilateral frontalis sling (Beard 1965). This, he found, results in a better cosmetic appearance and prevents the asymmetric lagophthalmos associated with the unilateral sling. The disadvantage of this procedure is the need to make a previously normal eyelid abnormal and subjecting the unaffected eyelid (and globe) to the standard risks of surgery. This may make the Beard procedure less appealing to parents of patients.
Bilateral sling without disinsertion of levator: In this modification of the classic Beard technique (sometimes called the Chicken Beard technique), a bilateral frontalis sling procedure is performed, but the unaffected levator is not disinserted. This has the advantage of maintaining better symmetry in downgaze than a unilateral sling, and does not require destruction of the normal levator. This may theoretically be more acceptable to parents of patients, as it does not disturb the integrity of the non-ptotic eyelid.
Super-maximal levator advancement: Some advocate a super-maximal (greater than 30 mm) resection of the levator muscle in these cases. While this approach can result in a better cosmetic outcome than a unilateral sling, problems such as conjunctival prolapse and significant lagophthalmos have been reported (Epstein 1984).
Whitnall's sling: An alternative to the super-maximal levator advancement is the Whitnall's sling in which the levator aponeurosis is removed and Whitnall's ligament and levator muscle are advanced to the superior aspect of the tarsal plate. This, too, can provide a better cosmetic result than a unilateral sling, and by keeping Whitnall's ligament intact, it has the added advantage of maintaining support of the lacrimal gland and temporal eyelid. Like the super-maximal resection, lagophthalmos and corneal exposure can occur after the Whitnall's sling procedure as well (Anderson 1990).
Marcus-Gunn Jaw Winking:
These cases represent a special type of unilateral ptosis in which treatment is based on both the amount of ptosis and the severity of the wink. If the wink is mild (i.e. if the lid height variation is minimal with jaw movement) and the ptosis is significant, the ptosis is corrected unilaterally, usually by advancing the levator. If, however, the wink is moderate to severe, the levator may have to be extirpated to eliminate the wink completely. The surgical options then are similar to those for unilateral ptosis with poor levator function discussed earlier (specificially, unilateral sling, bilateral levator extirpation with bilateral sling, or bilateral sling with preservation of the sound levator).
EPIDEMIOLOGY
- Presents at birth
- Usually non-progressive
- May have a familial component
SIGNS
- Unilateral or bilateral ptosis
- Poor levator function
- Poor eyelid crease
- Amblyopia/irregular astigmatism
SYMPTOMS
- Drooping of one or both eyelids
- Not associated with other ocular or systemic disorders
- No variation in eyelid position with jaw movement or ocular motility
TREATMENT
- Depends on amount of ptosis and levator function
- Frontalis sling (either unilateral versus bilateral) versus levator resection
- Observation is appropriate if there is no amblyopia and the patient has not adopted an abnormal head position
Gandhi N, Allen RC. Congenital Ptosis. EyeRounds.org. July 20, 2010; Available from: www.EyeRounds.org/ cases/114-Congenital-Ptosis.htm

Ophthalmic Atlas Images by EyeRounds.org, The University of Iowa are licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License.